Third Dose of COVID-19 Vaccine Increases Neutralizing Antibodies Against Omicron in Cancer Patients - Cancer Therapy Advisor

Comprehensive assessment of humoral response after Pfizer BNT162b2 mRNA Covid-19 vaccination: a three-case series

Vaccines | Free Full-Text | Quantitative Analysis of Anti-N and Anti-S Antibody Titers of SARS-CoV-2 Infection after the Third Dose of COVID-19 Vaccination
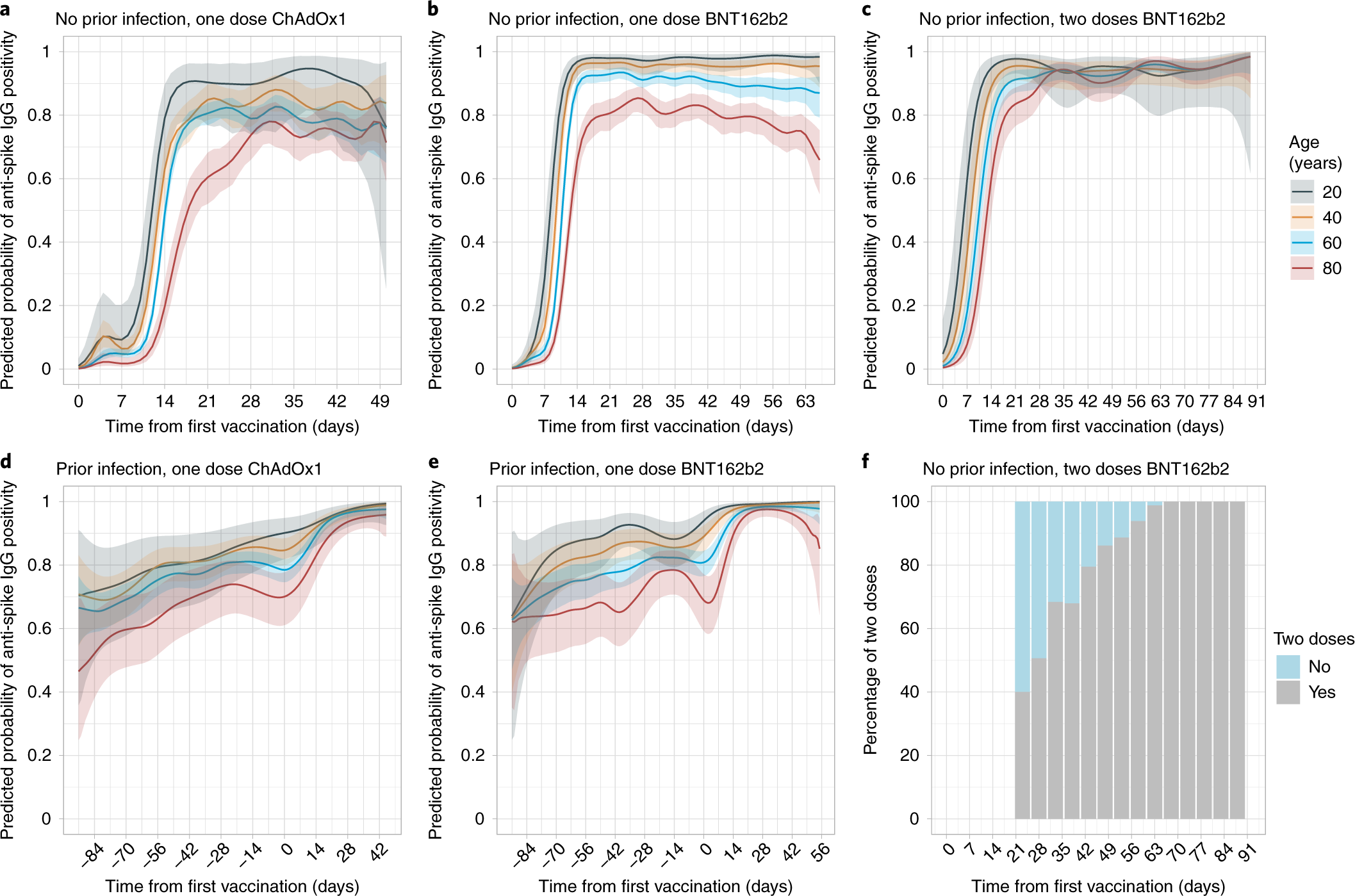
Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom | Nature Microbiology

SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis - ScienceDirect
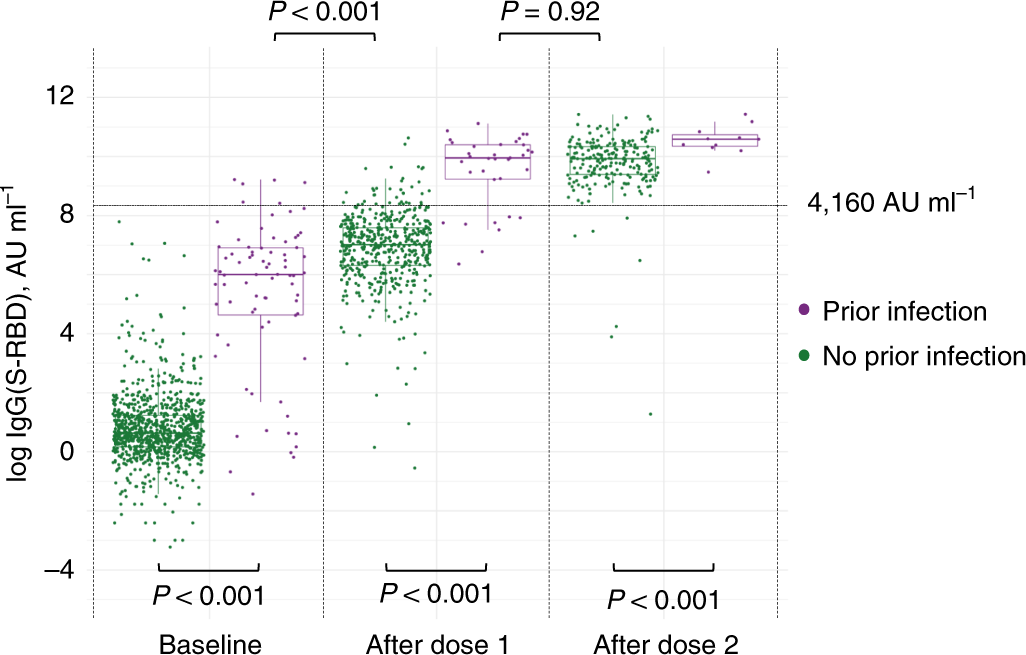
Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2 | Nature Medicine

Data shows greater, broader SARS-CoV-2 neutralizing antibodies with third dose of Pfizer/BioNTech vaccine

Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects | Microbiology Spectrum

Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT - The Lancet Haematology

Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors | Nature Medicine

Largest Study to Date Demonstrates Most Blood Cancer Patients Benefit From a Third Primary Dose of mRNA COVID-19 Vaccine | Leukemia and Lymphoma Society