TuraSkills shares tip for writing #Module 2.5 #Clinical overview #CTD overview #Common Technical Documents # CTD #Regul… | Technical writing, Writing tips, Writing
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience

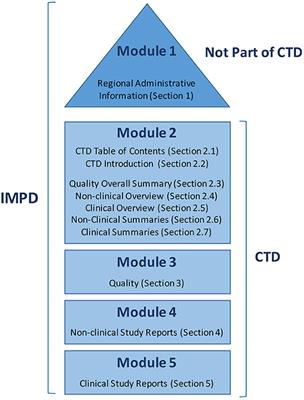
Schematic representation of the five modules in the Common Technical... | Download Scientific Diagram

ICH Public Meeting January 21, Fishers Lane, Room 1066 FDA PERSPECTIVE IMPLEMENTATION STATUS OF THE CTD Justina A. Molzon, M.S. Pharm., J.D. - ppt download

ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub

Schematic representation of the five modules in the Common Technical... | Download Scientific Diagram

Nonclinical Information in the Common Technical Document: Opportunities for Content Reuse Peggy Zorn, MPI Research Susan Mattano, Pfizer, Inc. - ppt download
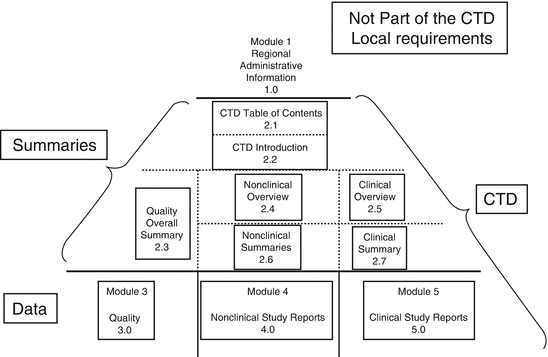
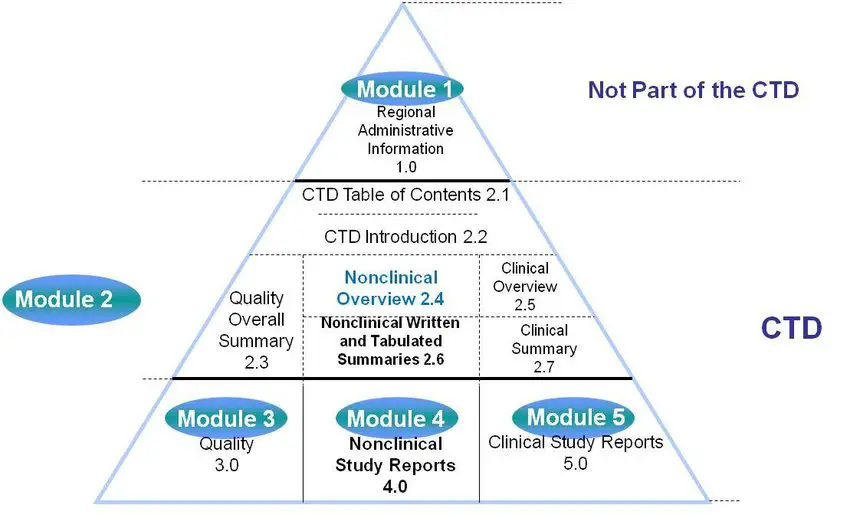
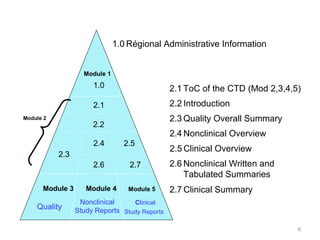
Annex 4] Organization of the Common Technical Document For the Registration of Pharmaceuticals for Human Use (With reference t

Last Update June 13 ' ToC of Module 1 or overall ToC, including Module ToC of the CTD (Mod 2,3,4,5) Module 1 Module 3Module 4Module ppt download

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH
WHO Guidelines on submission of documentation for the pilot procedure for prequalification of similar biotherapeutic products fo
Potential to Simplify the Writing of Submission Documents: Evaluation of Publicly Available Module 2 Documents in Drug Submissio

A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor
Transparency and information for decision making: patient engagement and publication of clinical data