Ivowen Ltd1 Ivowen Limited Preparation and Submission of a Traditional Herbal Medicinal Product Application. - ppt download
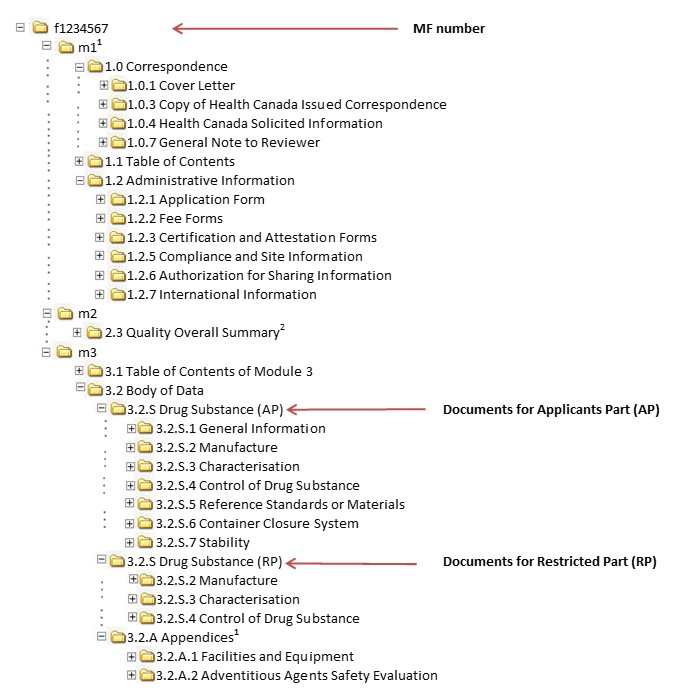
Progress Toward Standardization of Submissions with the Electronic Common Technical Document and the Evolving Standardization of

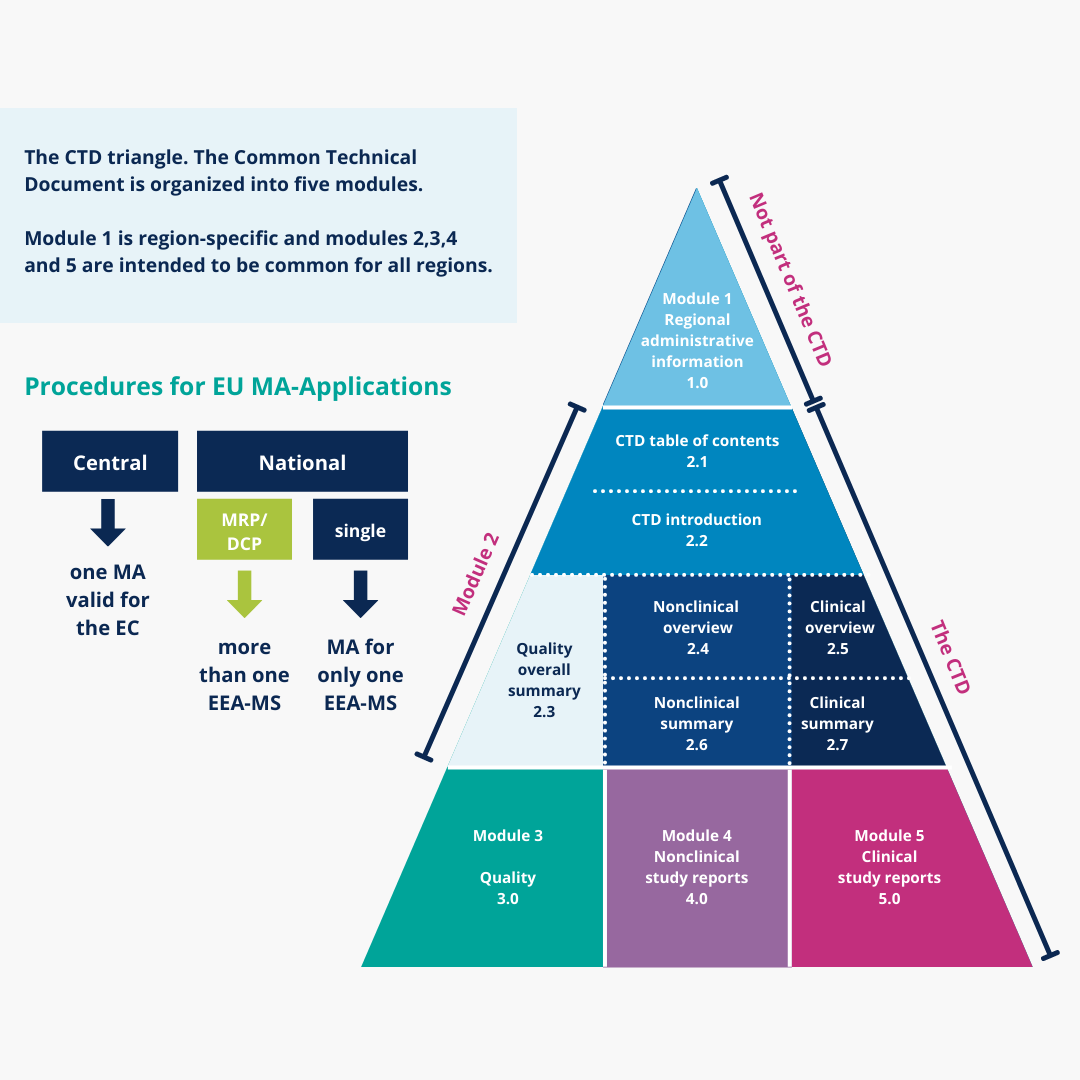
ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub
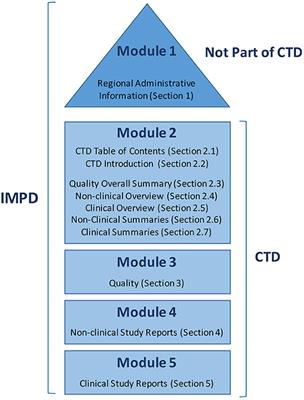
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience

Training Workshop on Pharmaceutical Development with a Focus on Paediatric Medicines / October |1 | Regulatory Requirement on Dossier of Medicinal. - ppt download