The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies - ScienceDirect

Strategies and Recommendations for Using a Data‐Driven and Risk‐Based Approach in the Selection of First‐in‐Human Starting Dose: An International Consortium for Innovation and Quality in Pharmaceutical Development (IQ) Assessment - Leach -

Strategies and Recommendations for Using a Data‐Driven and Risk‐Based Approach in the Selection of First‐in‐Human Starting Dose: An International Consortium for Innovation and Quality in Pharmaceutical Development (IQ) Assessment - Leach -

Harper's Weekly. genommen;, sagte Mabel, softlv. hersolf neben Mis: MABELS durch den Autor einer ausgeführt. Möchten ul. e: r - ich nehme Es liniii-li Zinn.-In-ich, aber es gibt keine H-ni. oin -
![PDF] Pharmacology-based toxicity assessment: towards quantitative risk prediction in humans. | Semantic Scholar PDF] Pharmacology-based toxicity assessment: towards quantitative risk prediction in humans. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/00bc482a71b2a1e0848268b81ada68b123f249d0/8-Figure5-1.png)
PDF] Pharmacology-based toxicity assessment: towards quantitative risk prediction in humans. | Semantic Scholar

Application of pharmacokinetics-pharmacodynamics/clinical response modeling and simulation for biologics drug development. | Semantic Scholar

The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies - ScienceDirect

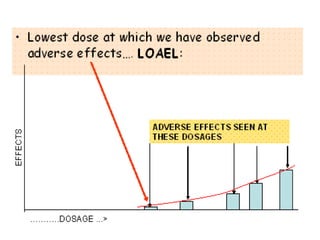
Receptor pharmacology or animal models for dose selection in humans? Bart Laurijssens Clinical Pharmacology Modelling & Simulation, GlaxoSmithKline, UK. - ppt download

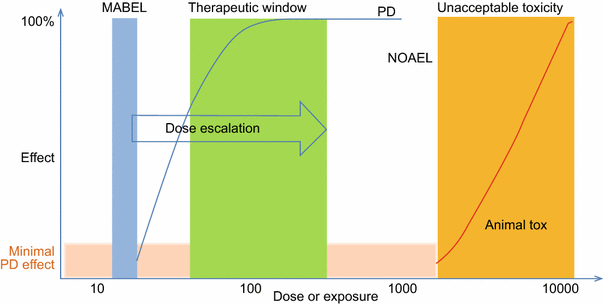
Calculation of the FIH dose from MABEL and NOAEL. The FIH dose from... | Download Scientific Diagram