A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor
eCTD - Neue Wege der elektronischen Einreichung - Vernetzung elektronischer regulatorischer Prozesse -
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan

ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

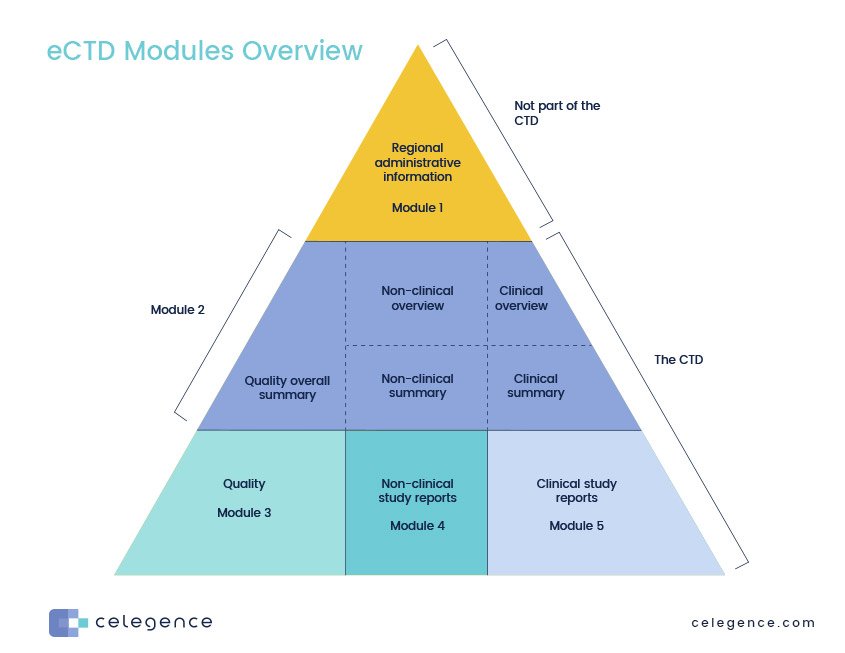
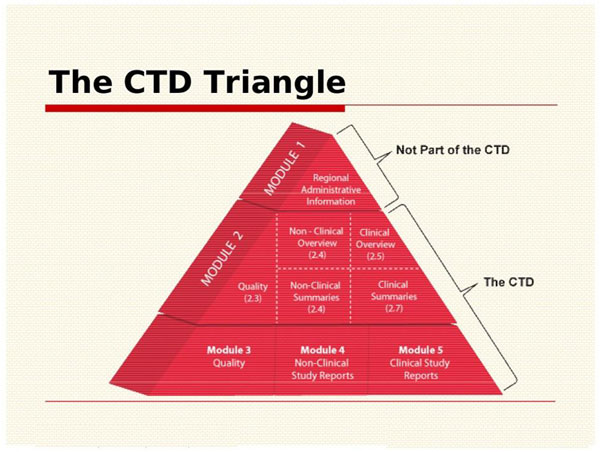
ClinProve - CTD Structure The Common Technical Document is divided into five modules: 1. Administrative and prescribing information 2. Overview and summary of modules 3 to 5 3. Quality (pharmaceutical documentation) 4.

Diagrammatic representation of the organization of follow-on biologics... | Download Scientific Diagram