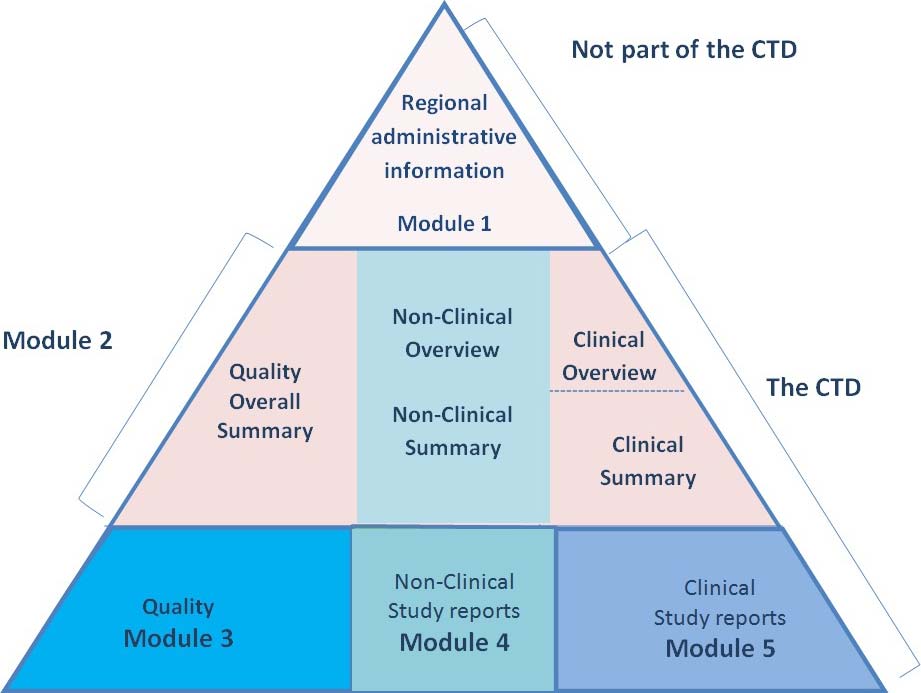
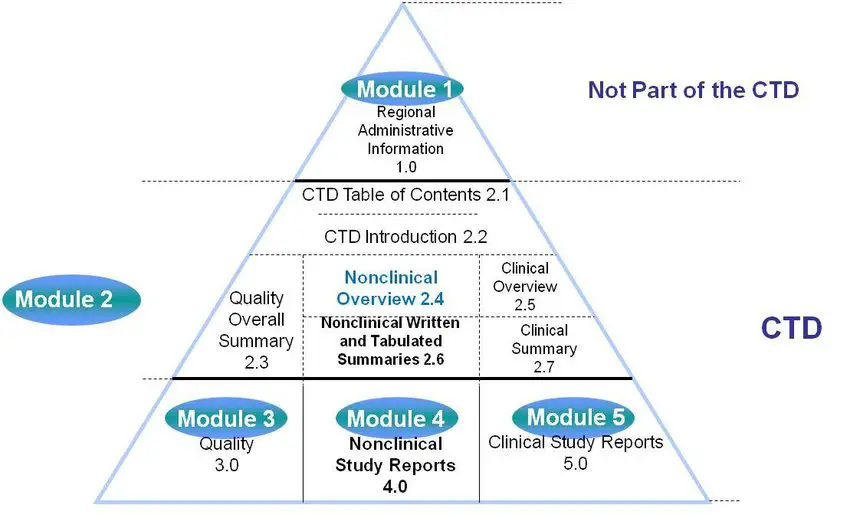
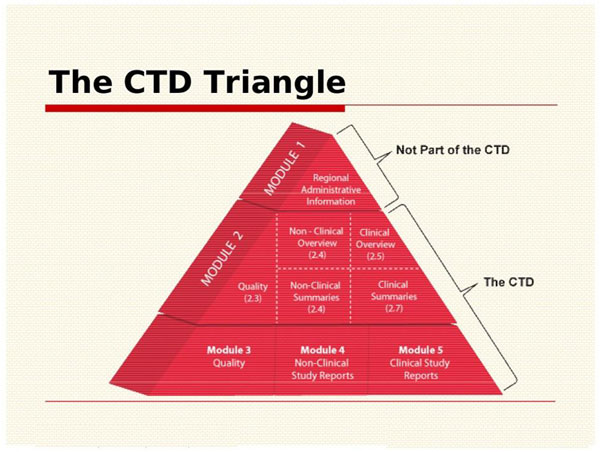
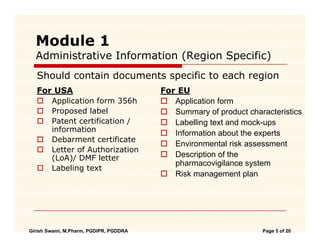
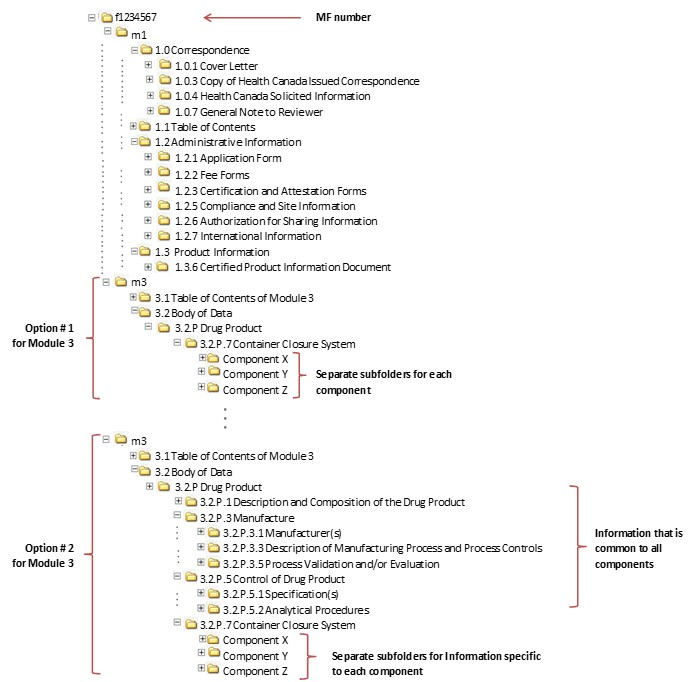
WHO Guidelines on submission of documentation for the pilot procedure for prequalification of similar biotherapeutic products fo

A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor