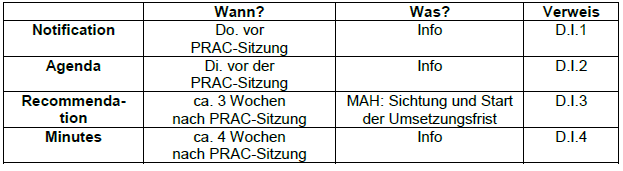
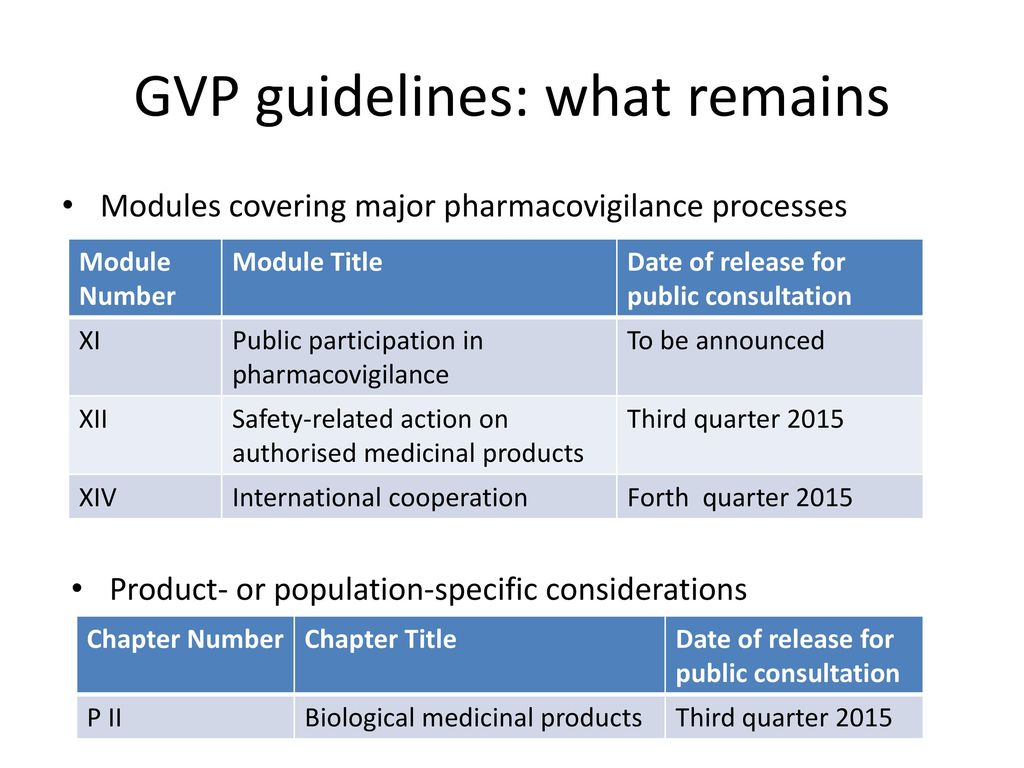
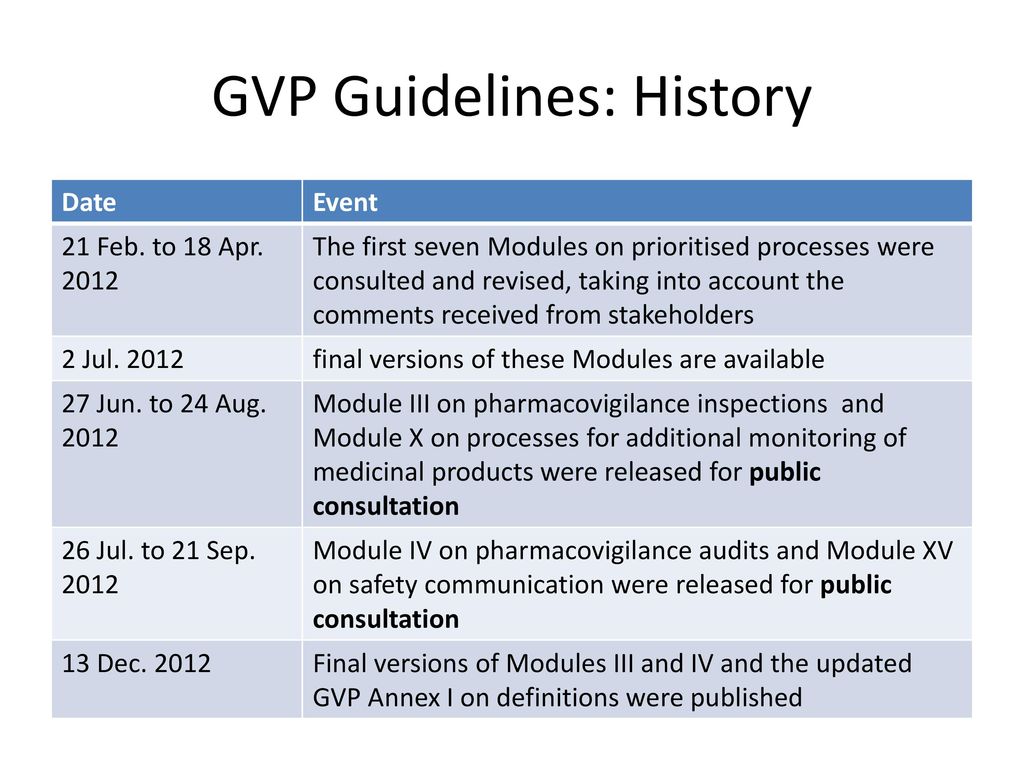
Comparison of the regulation for Good Pharmacovigilance Practice in the European Union and in the Eurasian Economic Union Wissen

Pilot III - Presentation 'Regulatory Support to Spanish Academia from STARS Core to Comprehensive Curriculum' - Stars

Guideline On Good Pharmacovigilance (GVP) - Module VIII - Post-Authorisation Safety Studies (Rev. 2) | PDF | Pharmacovigilance | Meta Analysis
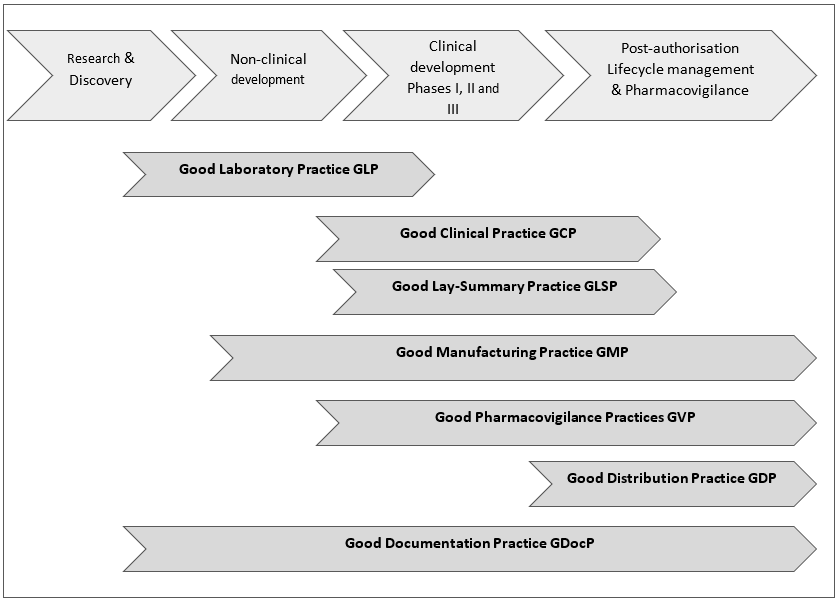
Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems








![EMA EXPECTATION WITH THE REVISED GUIDELINE OF RISK MANAGEMENT PLAN [GVP MODULE V] | NUJPS EMA EXPECTATION WITH THE REVISED GUIDELINE OF RISK MANAGEMENT PLAN [GVP MODULE V] | NUJPS](http://www.nujps.com/wp-content/uploads/2022/02/13.jpg)