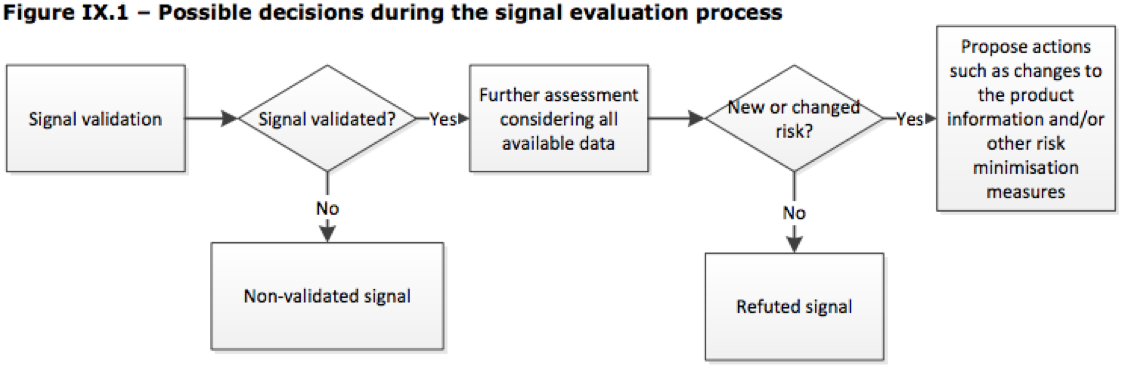
Guideline on good pharmacovigilance practices (GVP) - Module IX Addendum I – Methodological aspects of signal detection from s

Guideline On Good Pharmacovigilance Practices (GVP) Module IX - Signal Management (Rev. 1) | PDF | Pharmacovigilance | Clinical Trial

Impact of Changing Regulations and the Dynamic Nature of European Risk Management Plans for Human Medicines on the Lifecycle of Safety Concerns | SpringerLink

New EU PV legislation An overview of the new pharmacovigilance legislation in Europe. What do you need to know? 24 January 2013 Shelley Gandhi Director. - ppt download
The NEW EudraVigilance System and the electronic reporting of ICSRs in the ISO/ICH E2B(R3) format: Hands-on Training Course

Signal Detection & Management: GVP IX (Clinical Research and Drug Safety Pharmacovigilance, Band 3) : . Net, Pro - Career: Amazon.de: Bücher