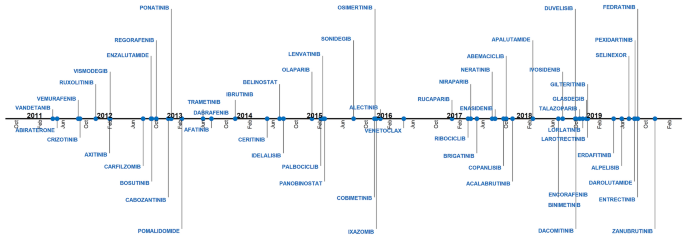
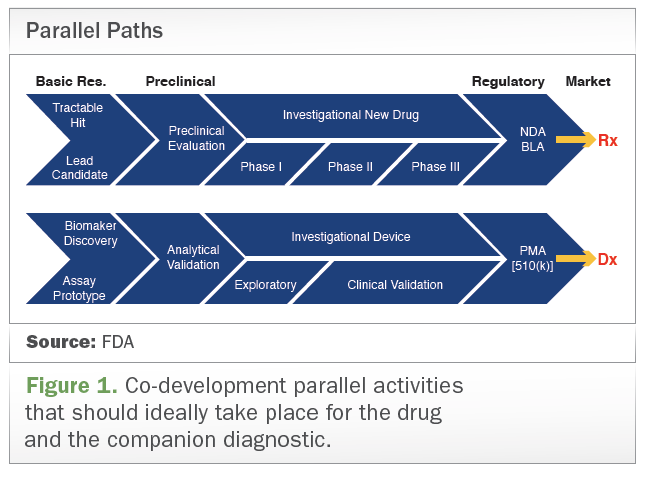
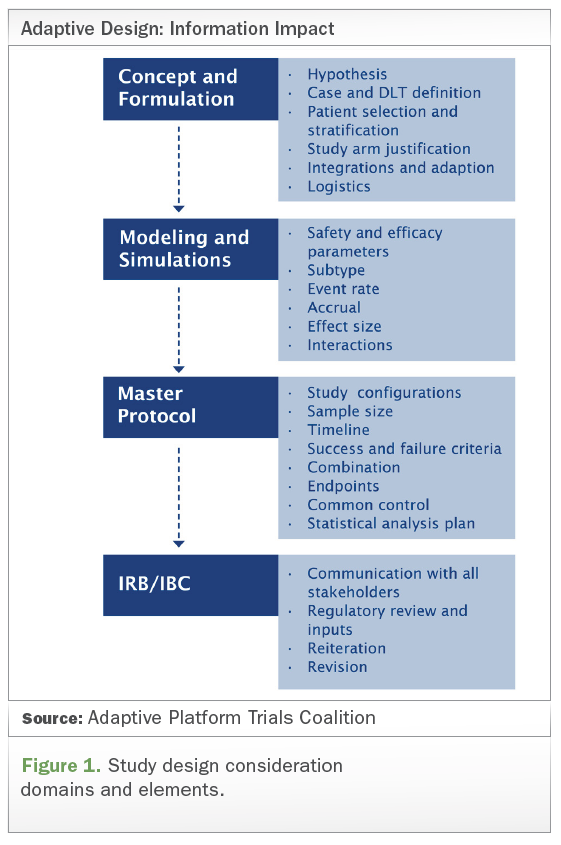
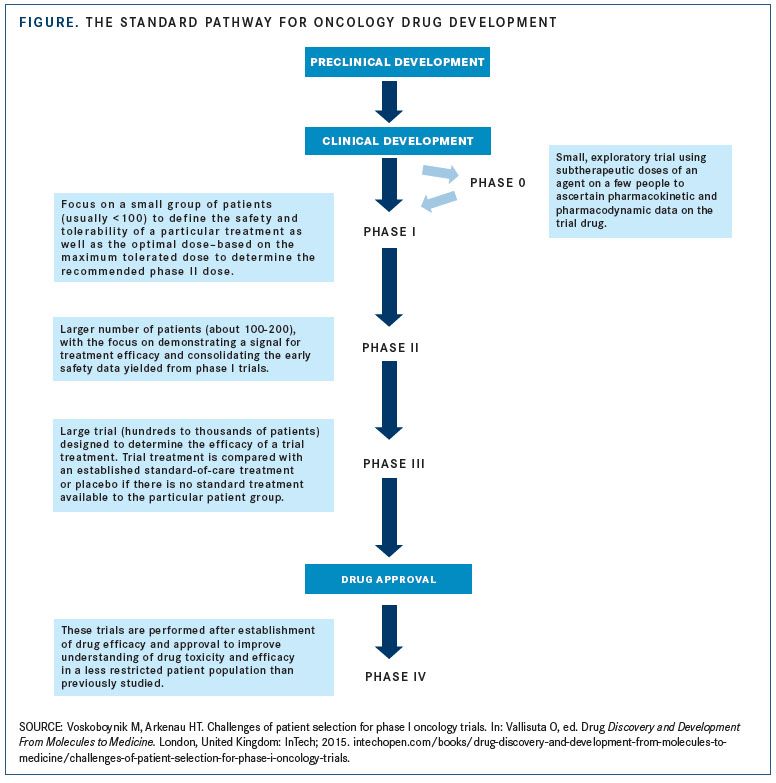
1 US FDA general guide for FIH dose selection for a cytotoxic agent and... | Download Scientific Diagram

FDA draft guidance aims to expedite first-in-human clinical trials for oncology drugs and biologics - Pearl Pathways
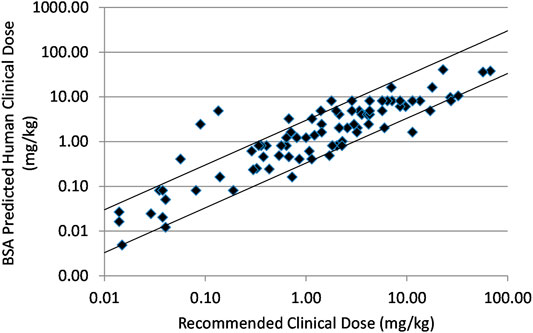
Frontiers | Predicting Approximate Clinically Effective Doses in Oncology Using Preclinical Efficacy and Body Surface Area Conversion: A Retrospective Analysis

Design and Conduct Considerations for First‐in‐Human Trials - Shen - 2019 - Clinical and Translational Science - Wiley Online Library

Twitter \ Tatiana Prowell, MD على تويتر: "Tweet 1/2❗️ FDA Guidance on Conduct of #ClinicalTrials of Med Products during #COVID19 Pandemic for Industry, Investigators, & IRBs. Participant safety #1. Discusses protocol chng/violations,

Starting dose selection and dose escalation for oncology small molecule first-in-patient trials: learnings from a survey of FDA-approved drugs | SpringerLink
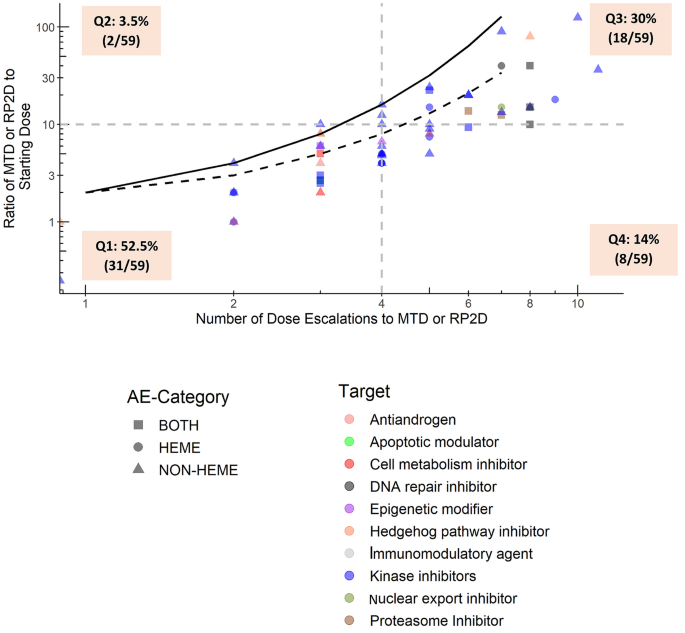
Starting dose selection and dose escalation for oncology small molecule first-in-patient trials: learnings from a survey of FDA-approved drugs | SpringerLink

Moving Beyond 3+3: The Future of Clinical Trial Design | American Society of Clinical Oncology Educational Book
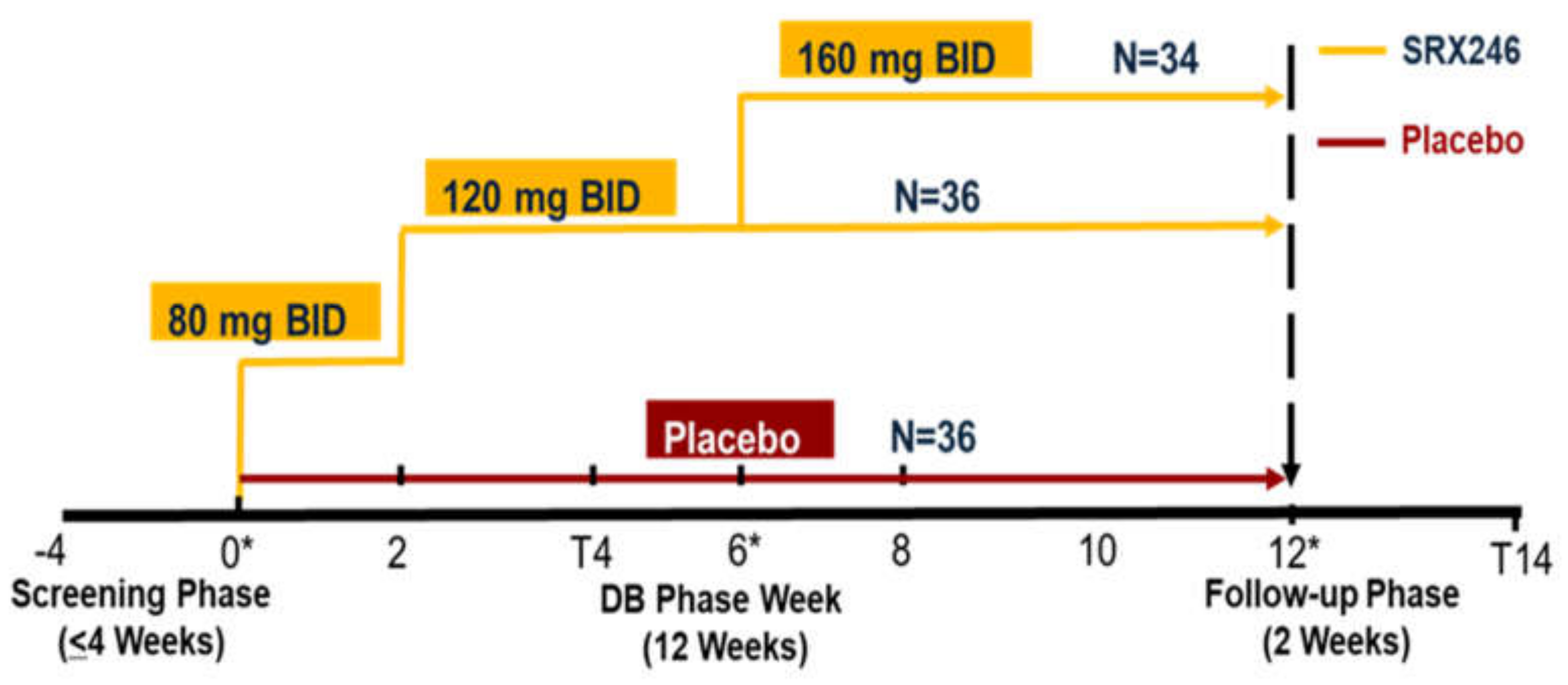
JCM | Free Full-Text | Safety and Tolerability of SRX246, a Vasopressin 1a Antagonist, in Irritable Huntington's Disease Patients—A Randomized Phase 2 Clinical Trial

Dose Finding in the Clinical Development of 60 US Food and Drug Administration–Approved Drugs Compared With Learning vs. Confirming Recommendations - Lyauk - 2019 - Clinical and Translational Science - Wiley Online Library

Moving Beyond 3+3: The Future of Clinical Trial Design | American Society of Clinical Oncology Educational Book