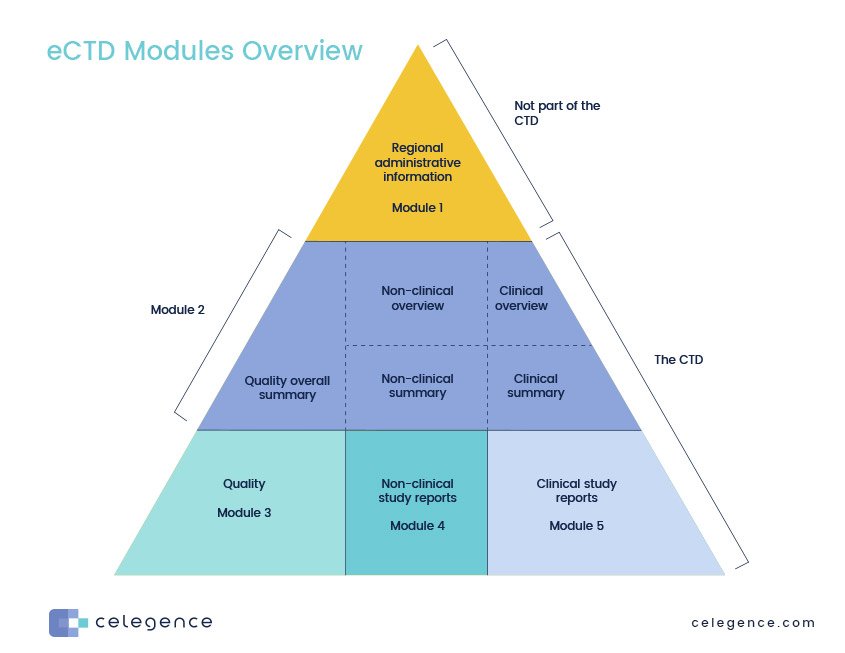
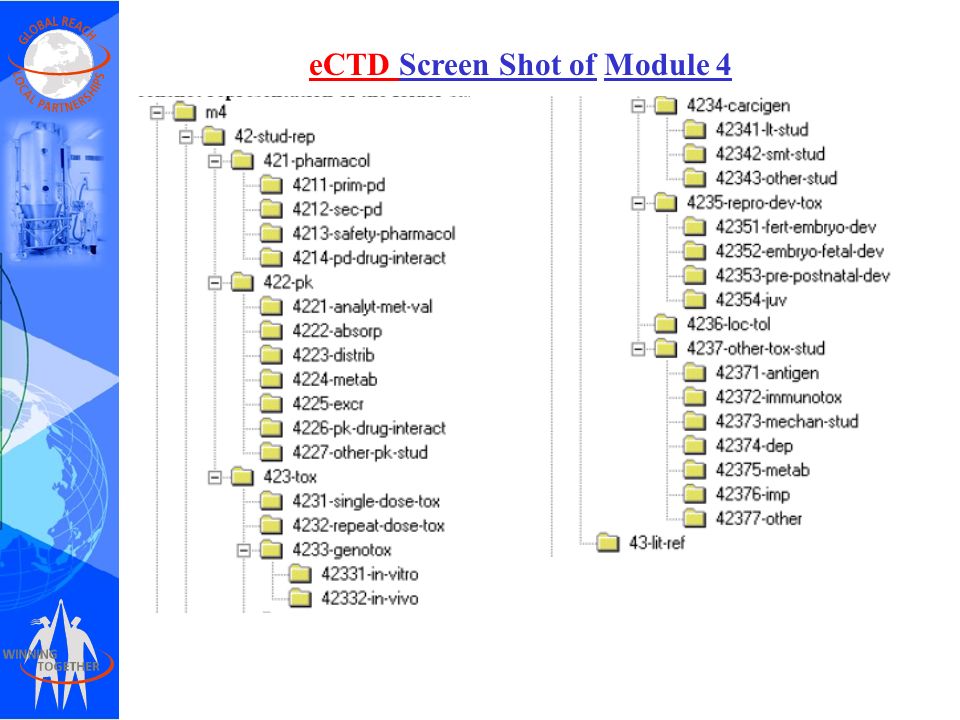
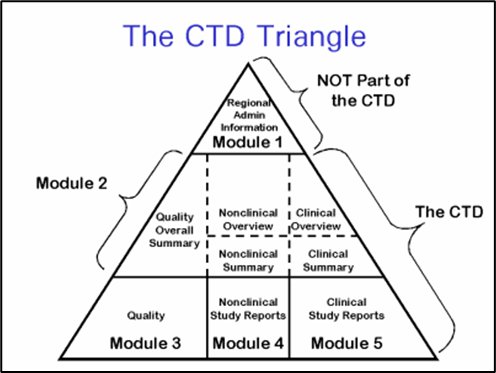
The Common Technical Document Elements (Source: ?Implementation of the... | Download Scientific Diagram

The Common Technical Document Elements (Source: ìImplementation of the... | Download Scientific Diagram

ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH