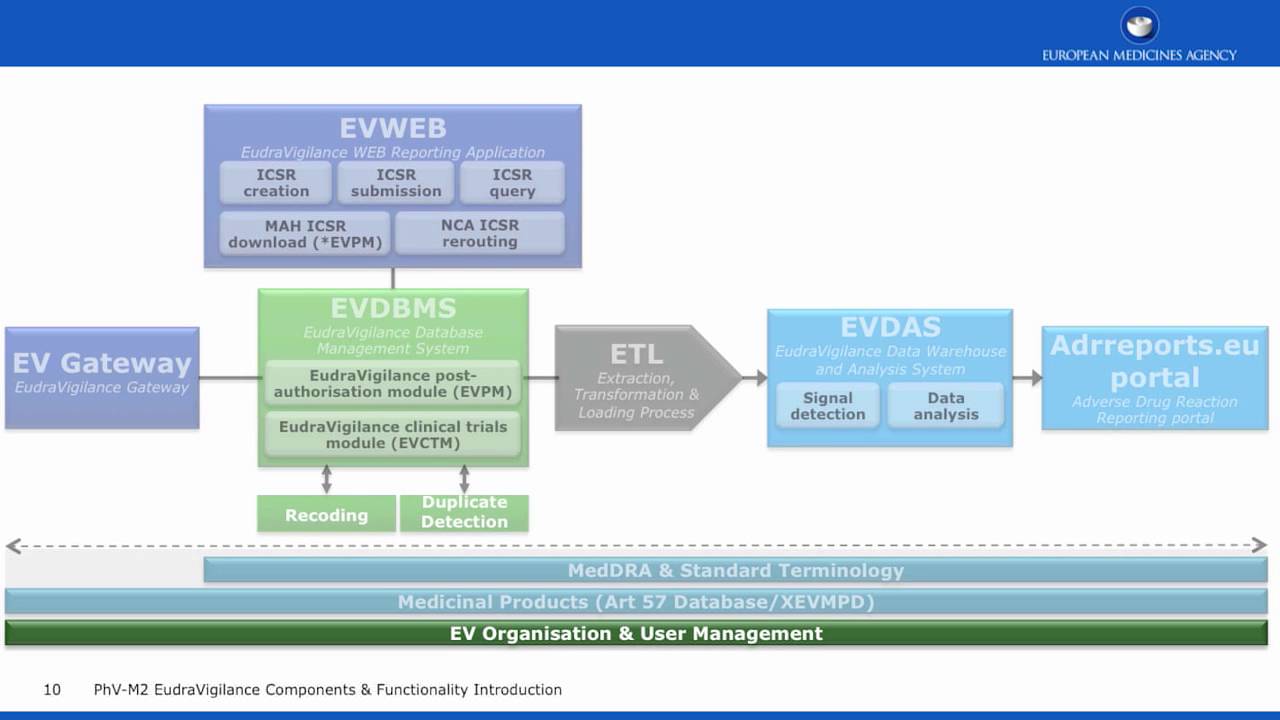
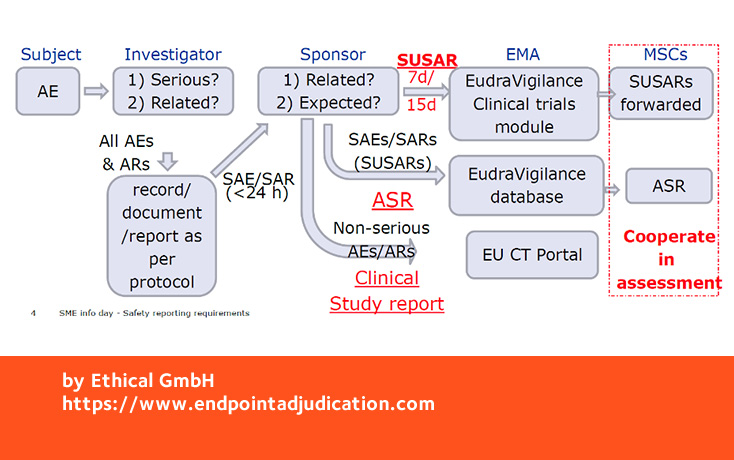
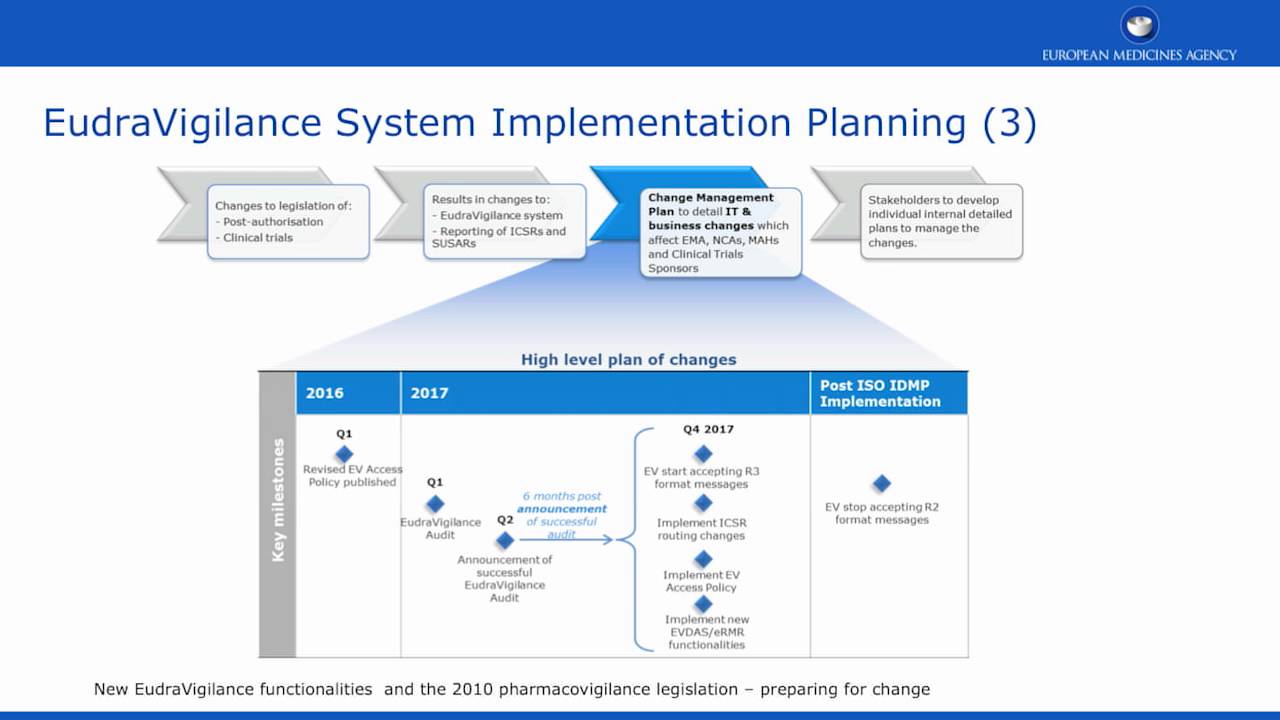
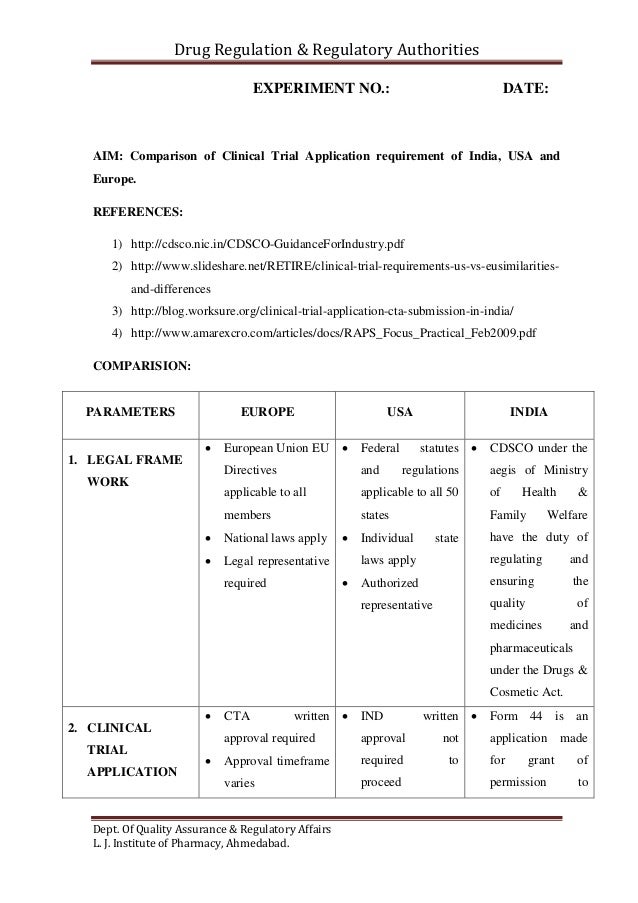
Grundzüge des neuen Genehmigungsverfahrens für klinische Arzneimittelprüfungen im Rahmen der Verordnung (EU) Nr. 536/2014 und der Zusammenarbeit zwischen den Mitgliedstaaten | SpringerLink

Teva Pharmaceuticals Europe 02 October 2007 Pharmacovigilance and electronic reporting Background and procedures Wendy Huisman EU QP Teva Pharmaceuticals. - ppt download