![Blog] EUDAMED – die Schlüsselrolle jedes Moduls - Pharma Serialization, Aggregation and Track and Trace Software von SoftGroup Blog] EUDAMED – die Schlüsselrolle jedes Moduls - Pharma Serialization, Aggregation and Track and Trace Software von SoftGroup](https://www.softgroup.eu/wp-content/uploads/2021/06/EUDAMED-2.png)
Blog] EUDAMED – die Schlüsselrolle jedes Moduls - Pharma Serialization, Aggregation and Track and Trace Software von SoftGroup
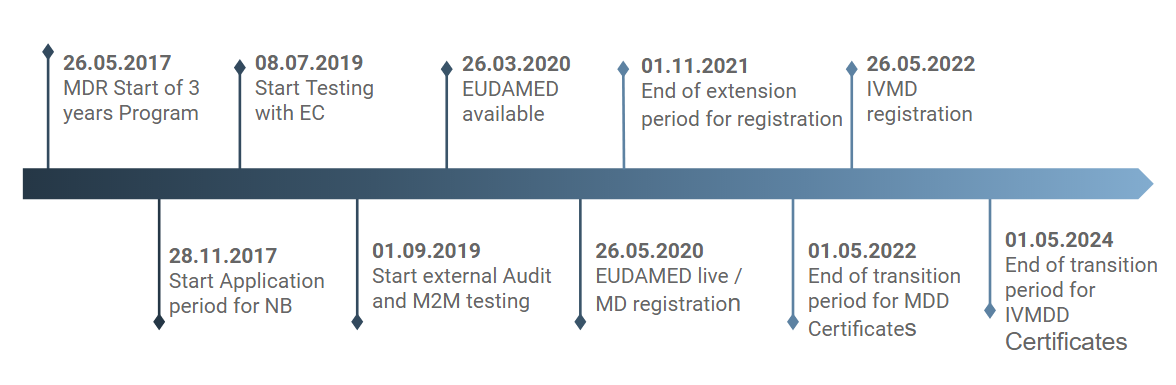
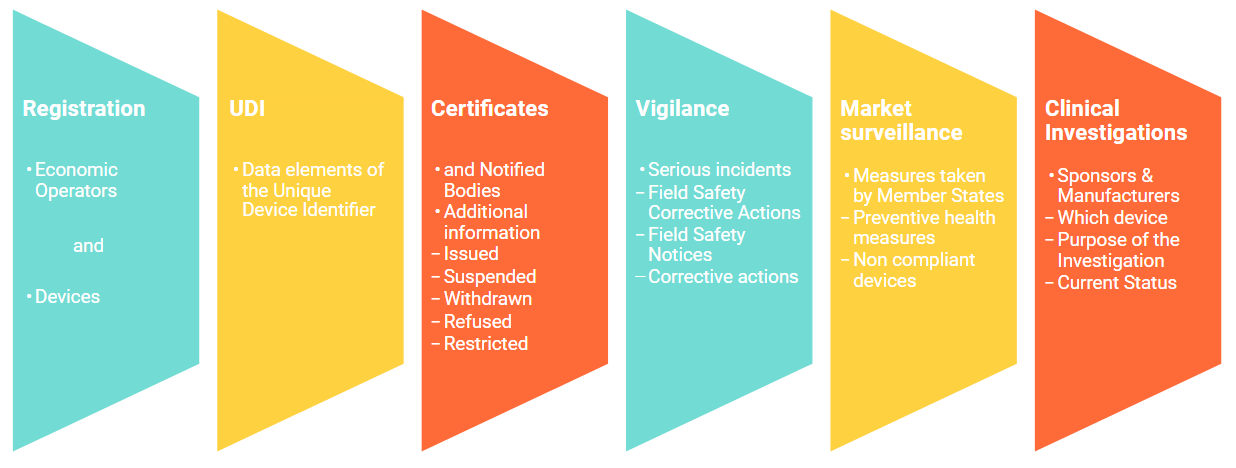
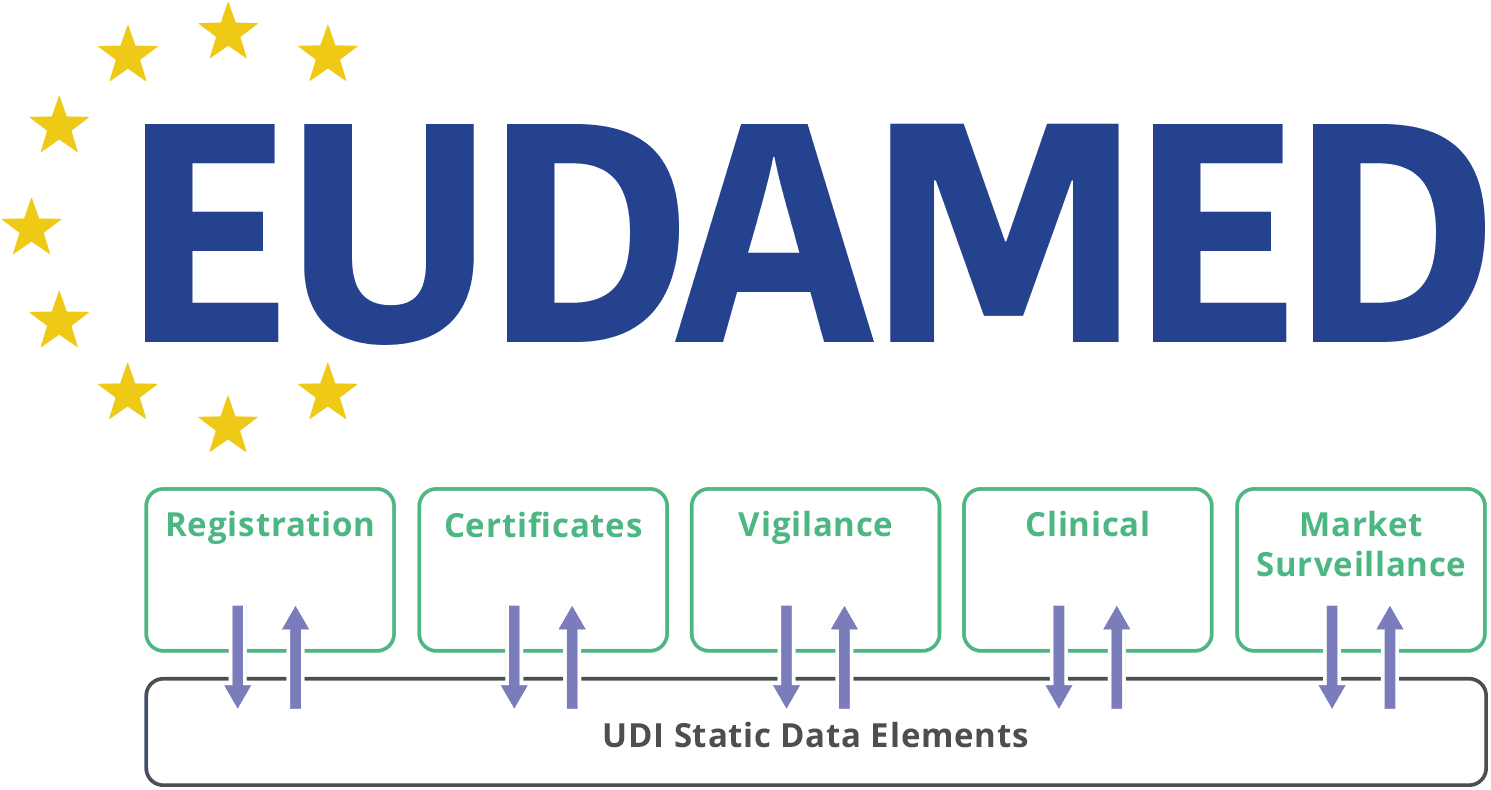
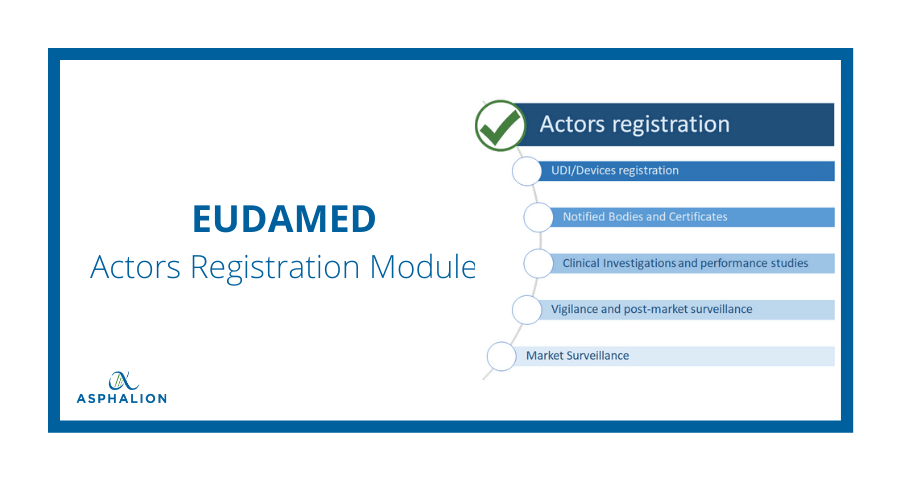
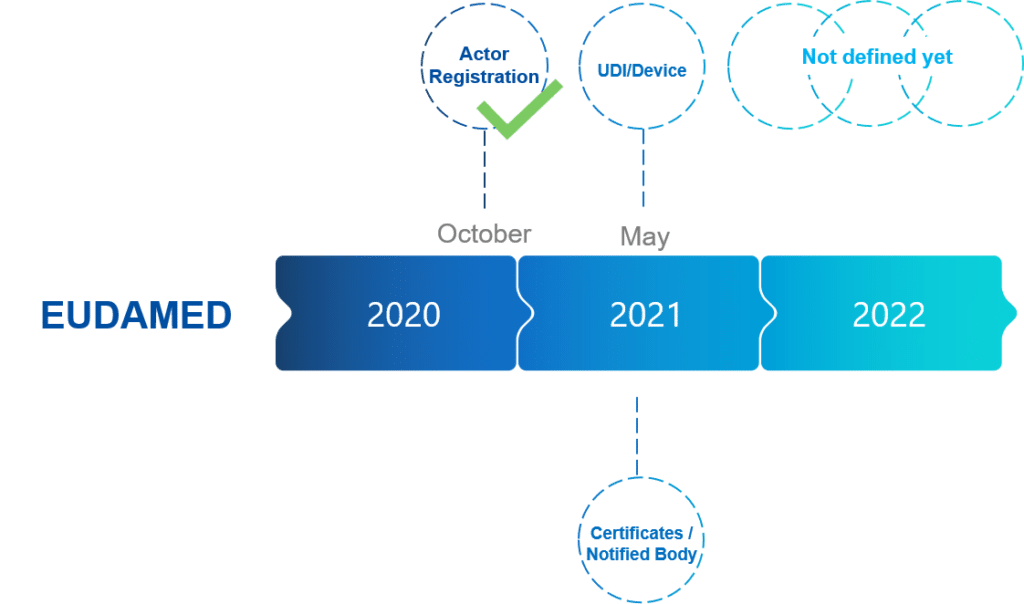
Functional specifications for the European Database on Medical Devices ( EUDAMED) - to be audited (only for Minimum Viable Pro