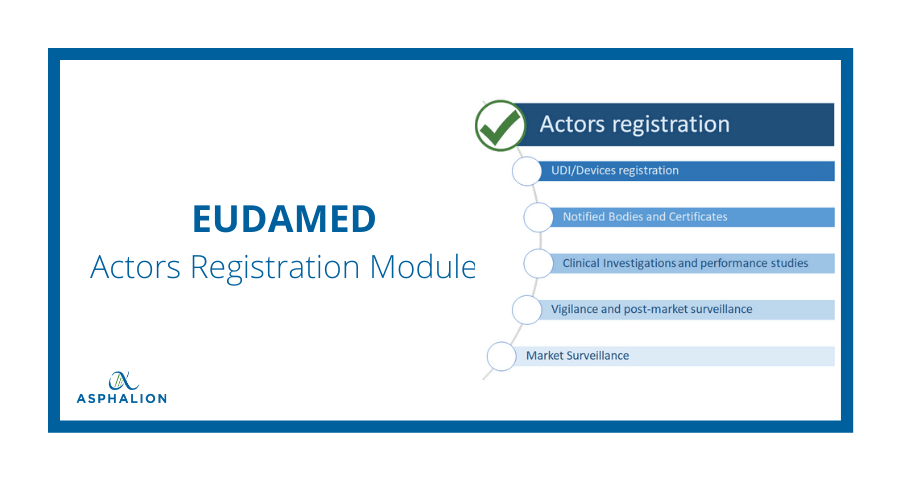
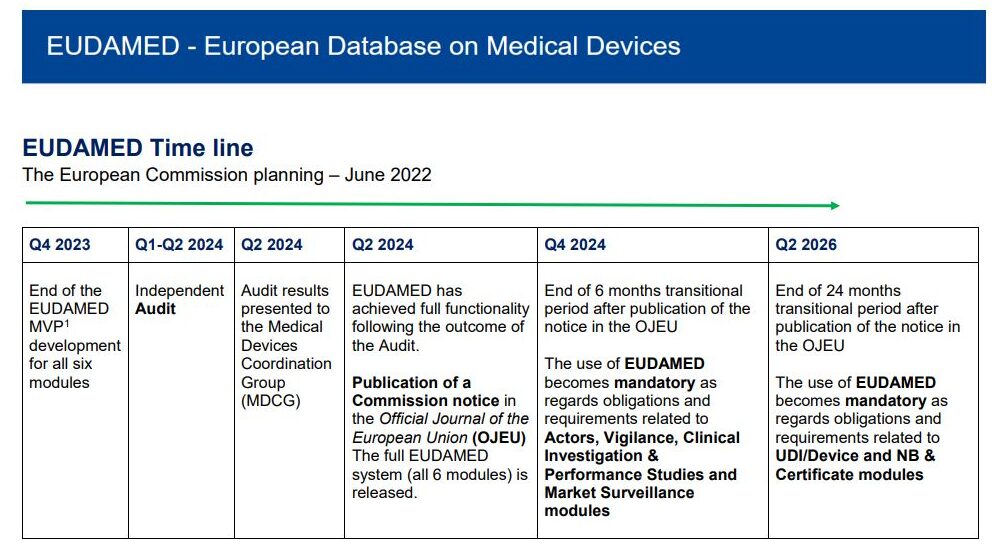
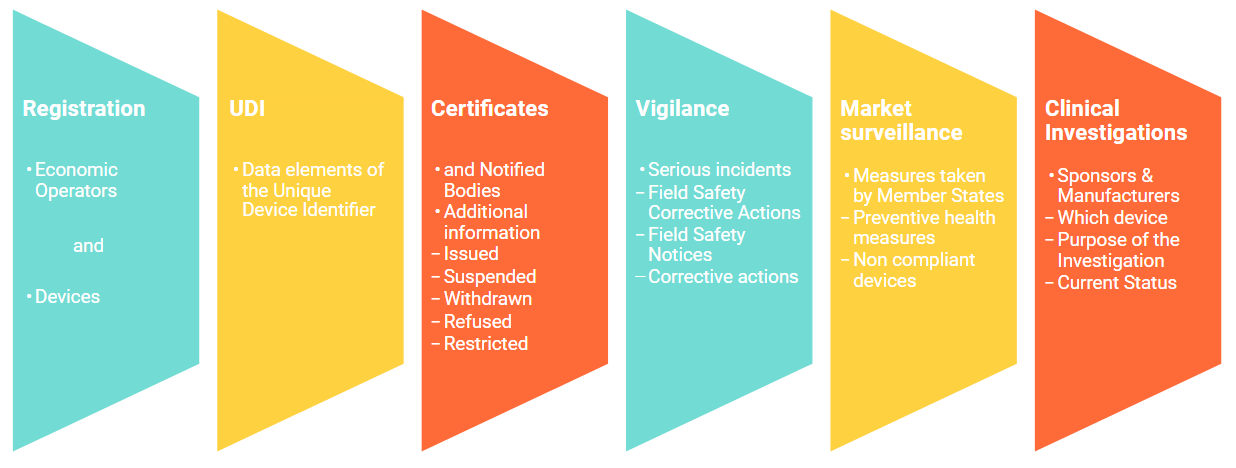
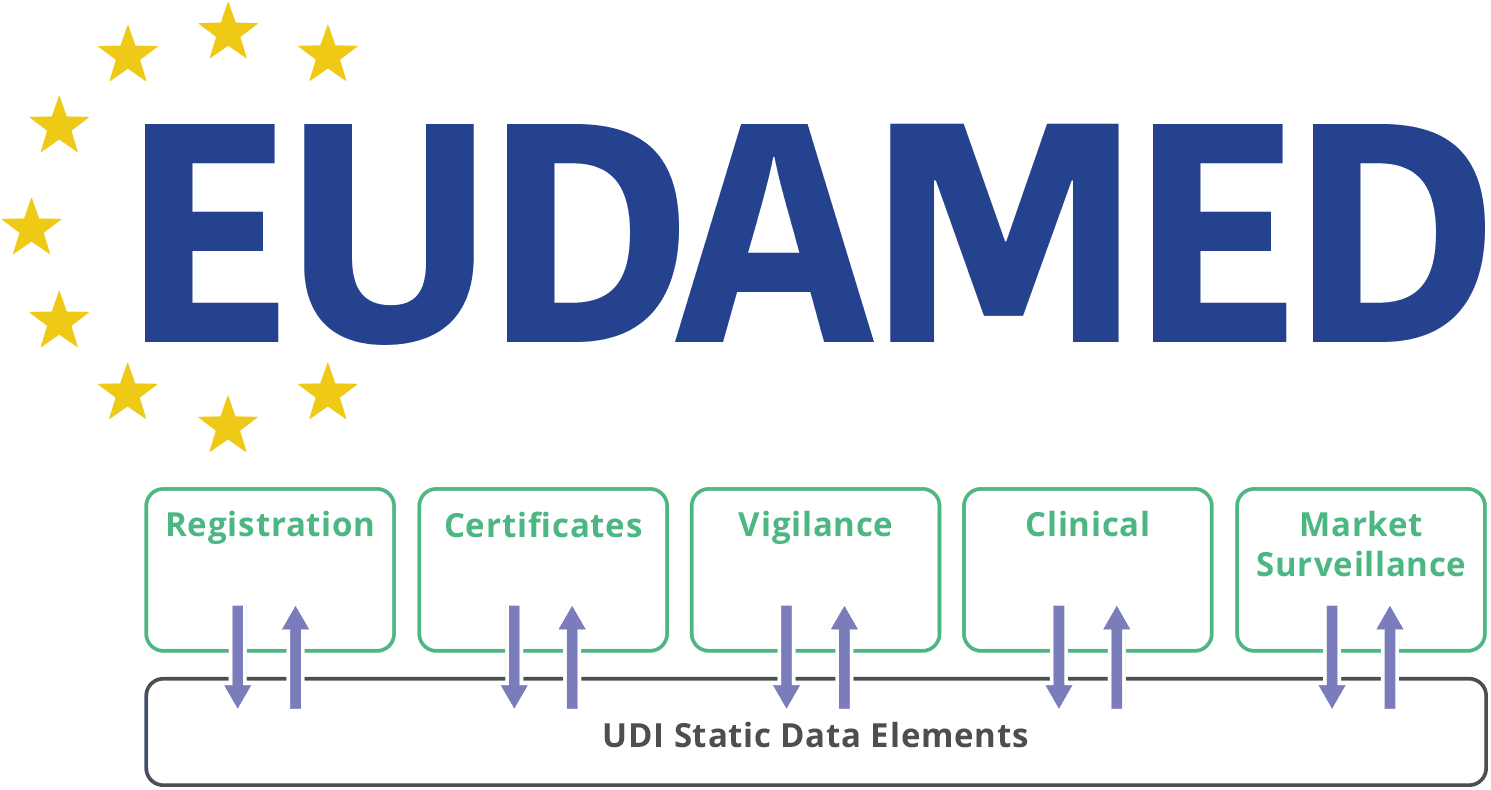
Eudamed modules for UDI, Notified Body certifications now live for medical device, IVD economic operators
Aktuelle Themen aus der EU-Mitarbeit (im Speziellen MDCG – IVD WG) und Herausforderungen der IVDR Implementierung aus Sicht de
![Blog] EUDAMED – the key role of each module - Pharma Serialization, Aggregation and Track and Trace Software by SoftGroup Blog] EUDAMED – the key role of each module - Pharma Serialization, Aggregation and Track and Trace Software by SoftGroup](https://34.118.101.194/wp-content/uploads/2021/06/EUDAMED-2.png)
Blog] EUDAMED – the key role of each module - Pharma Serialization, Aggregation and Track and Trace Software by SoftGroup