Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience
Guideline on the use of the CTD format in the preparation of a registration application for traditional herbal medicinal product

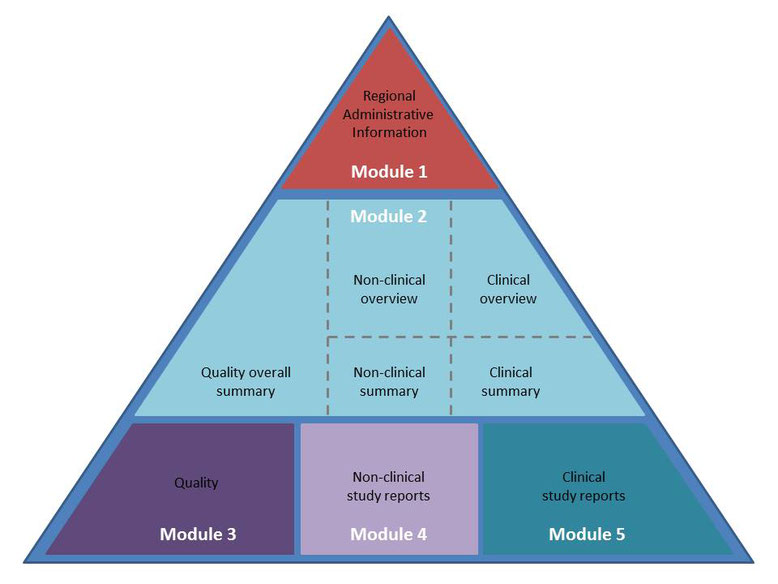
Representation of the components of the CTD. The nonclinical components... | Download Scientific Diagram










(1).png)

