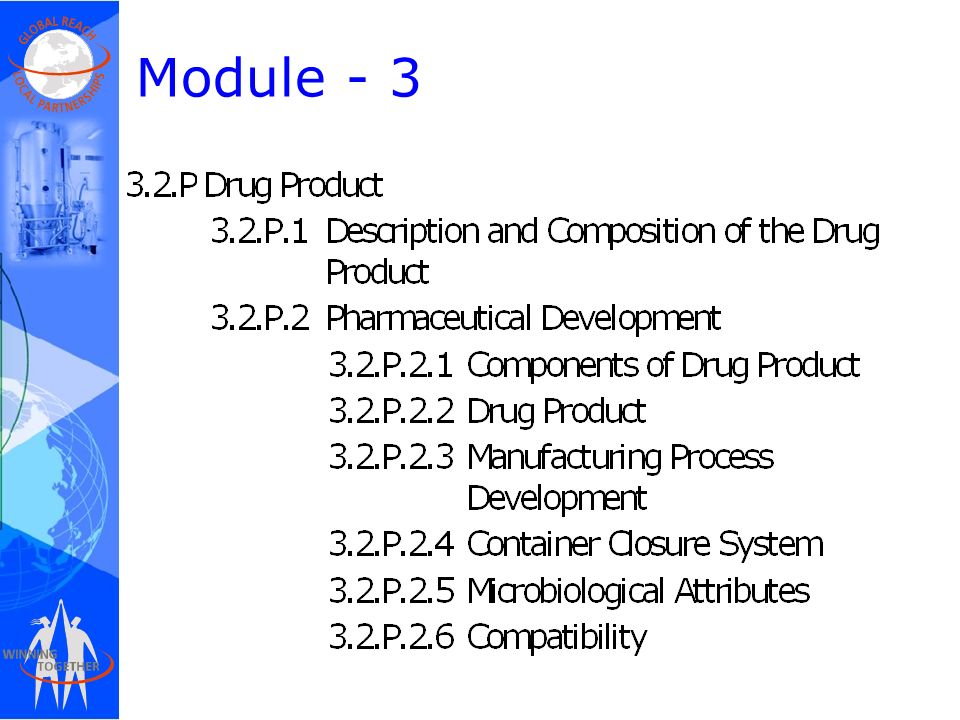
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan
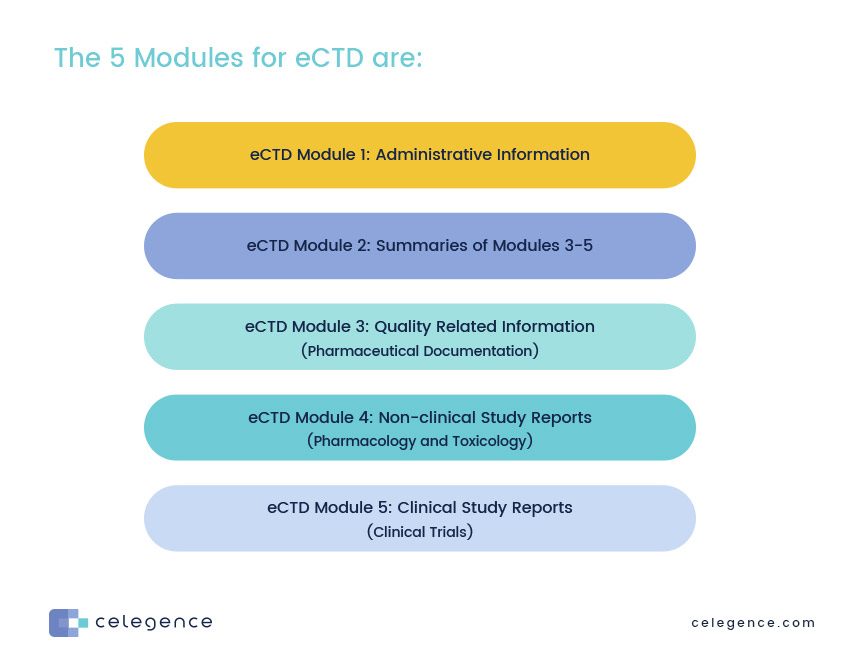
ELECTRONIC COMMON TECHNICAL DOCUMENT (eCTD): A REVIEW OF HISTORY, BENEFITS OF IMPLEMENTING, CHALLENGES, MODULES, RISKS INVOLVED
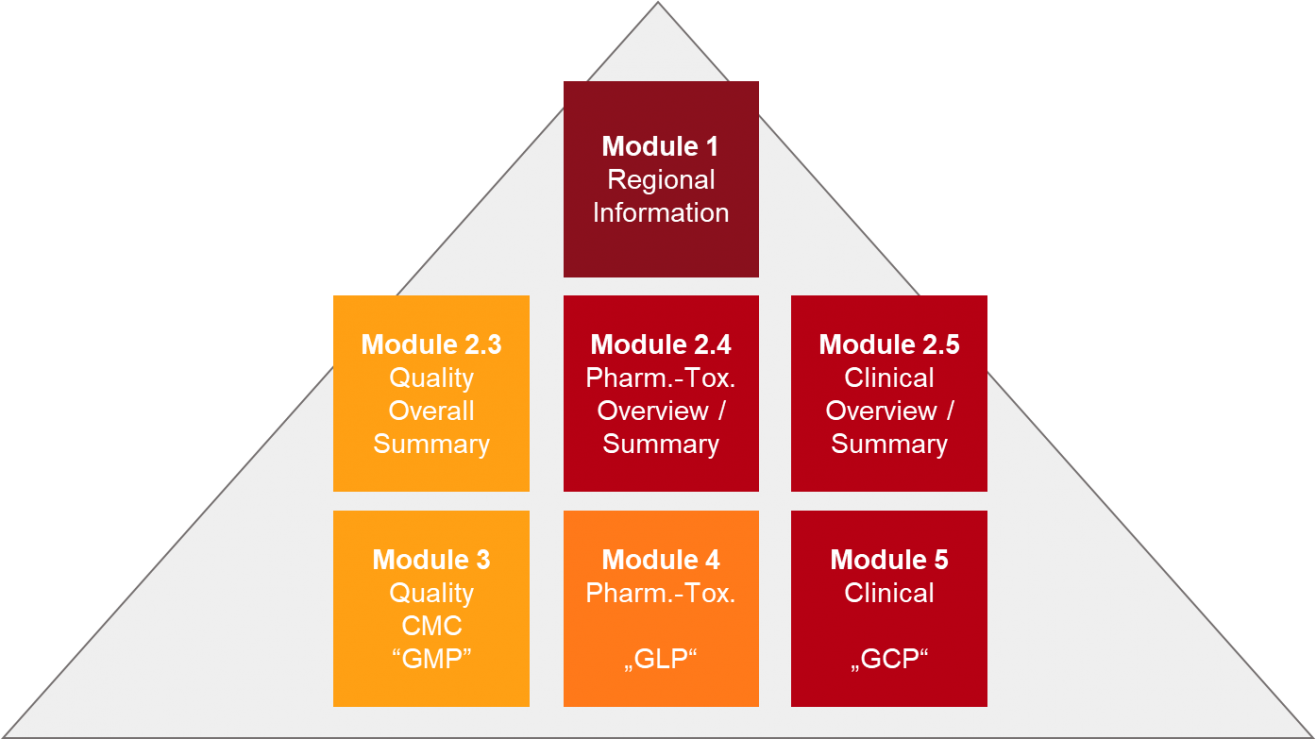
Exposé zum Thema: eCTD - Neue Wege der elektronischen Arzneimittelzulassung und die Vernetzung elektronischer regulatorischer P

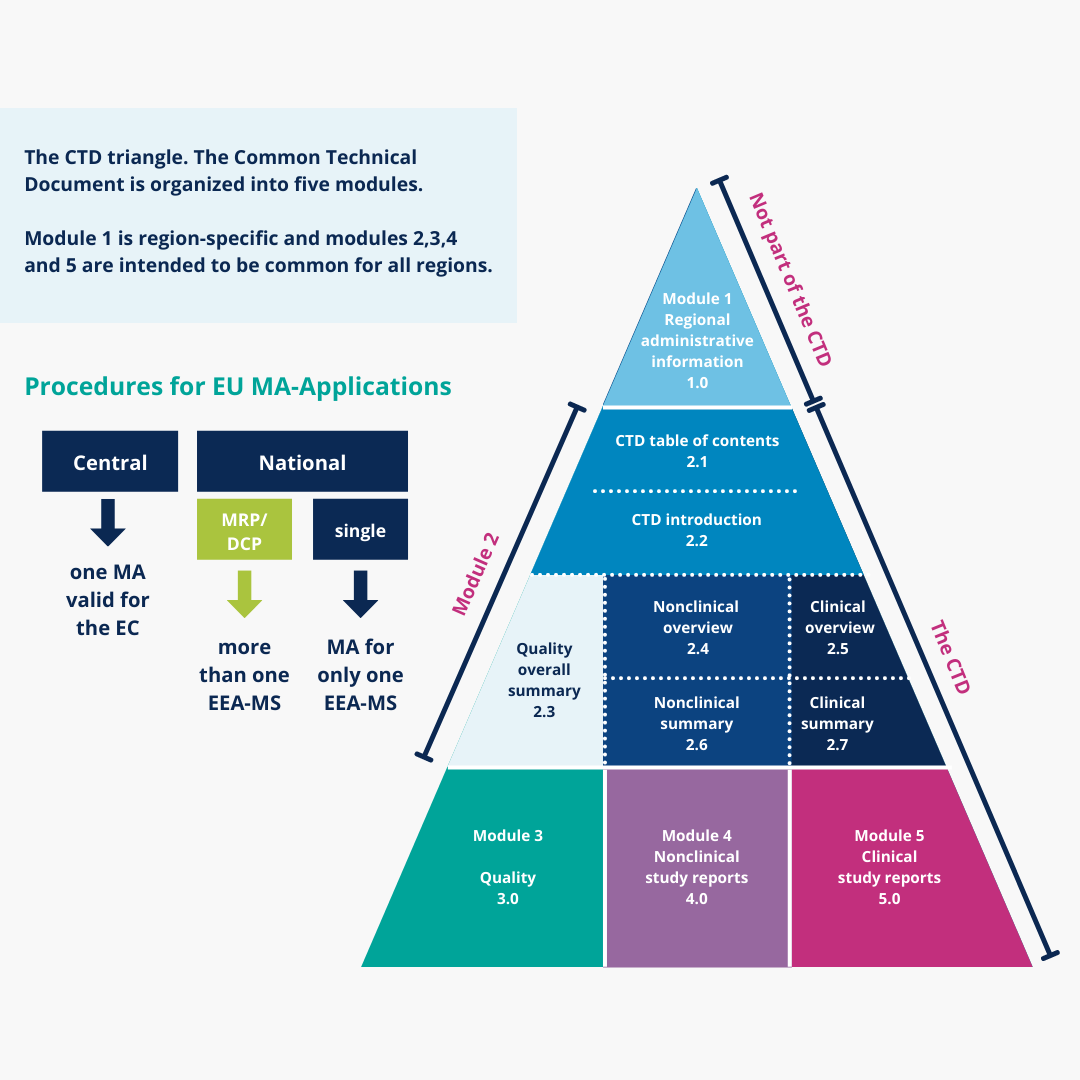
The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH
eCTD - Neue Wege der elektronischen Einreichung - Vernetzung elektronischer regulatorischer Prozesse -