Learning Module: Developing Your Teaching Dossier - Lesson 4 | Taylor Institute for Teaching and Learning | University of Calgary
Hinweise zur Einreichung von Registrierungsanträgen nach § 38 AMG beim Bundesinstitut für Arzneimittel und Medizinprodukte

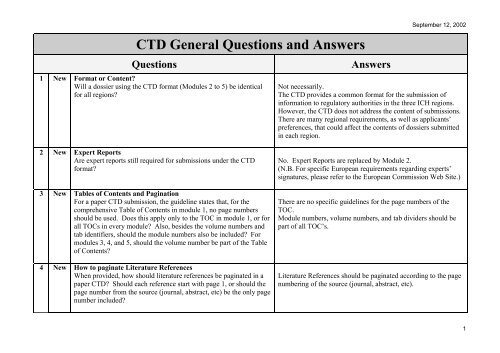
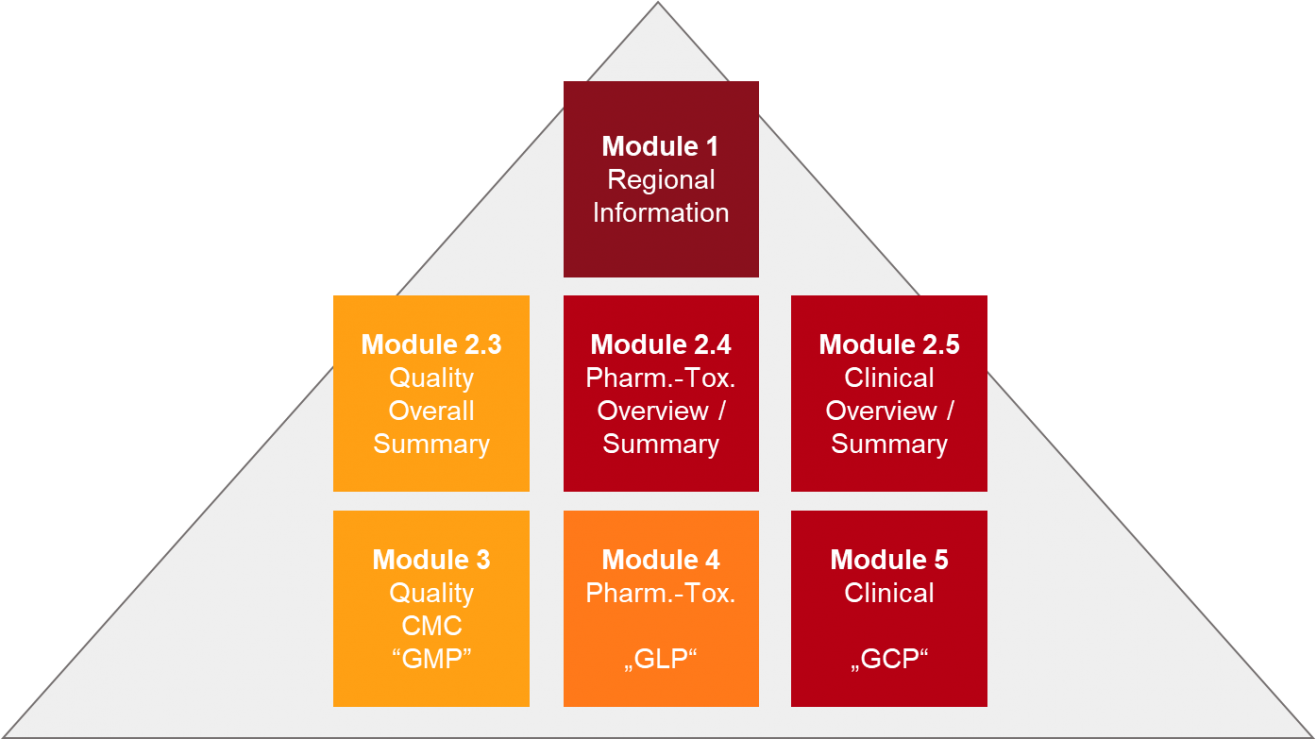
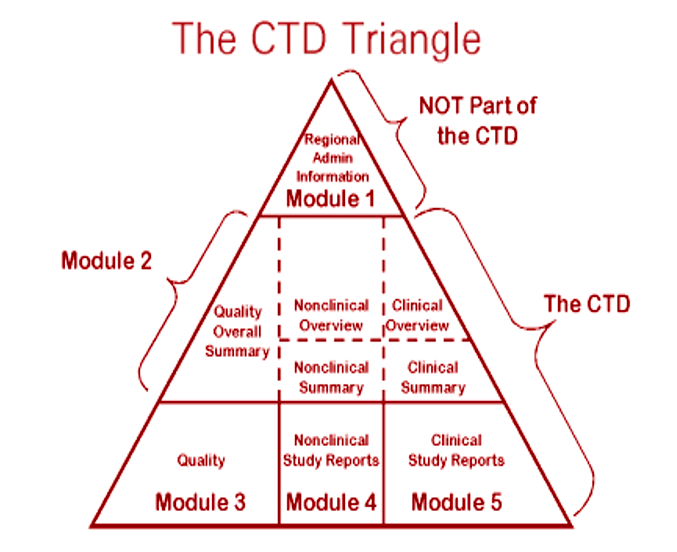
The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

Klett Sprachen Dossier Le Maghreb in Köln - Porz | Fachbücher für Schule & Studium gebraucht kaufen | eBay Kleinanzeigen
WHO Guidelines on submission of documentation for the pilot procedure for prequalification of similar biotherapeutic products fo

F5 BIGIP Loadbalancer: Wie wird das Produkt aktiviert, was ist ein Dossier und Registration Key - krakovic.de

9782311203387: DEASS DC1 Le dossier de pratiques professionnelles modules 4e edt (Itinéraires pro modules) (French Edition) - MOLINA, YVETTE: 231120338X - AbeBooks