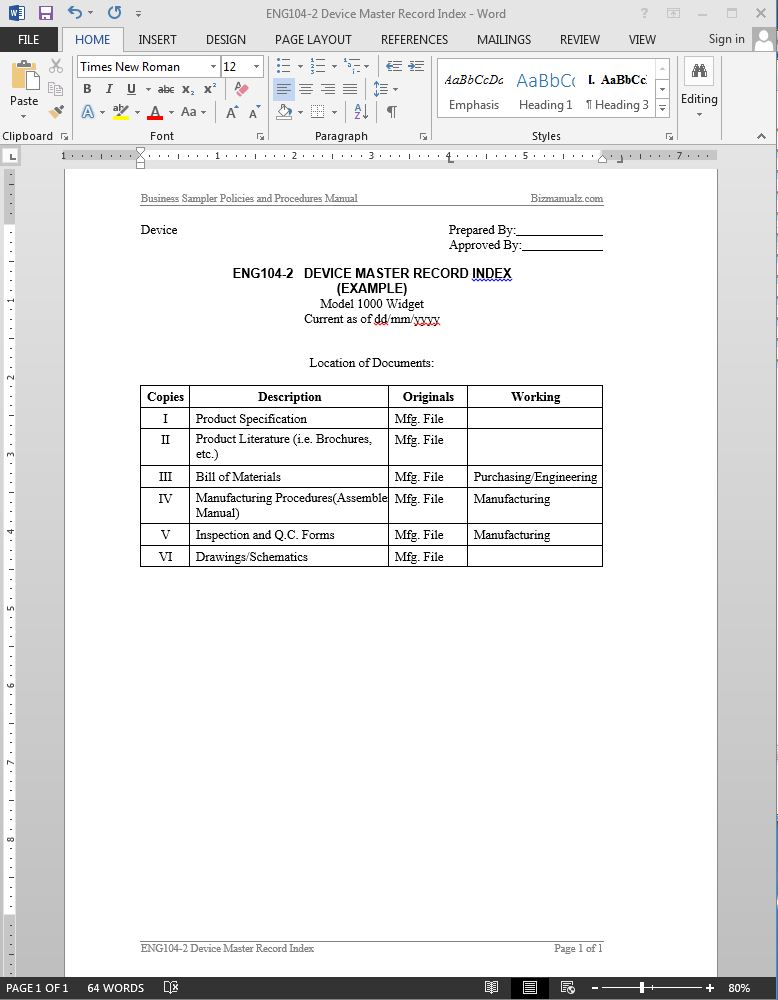
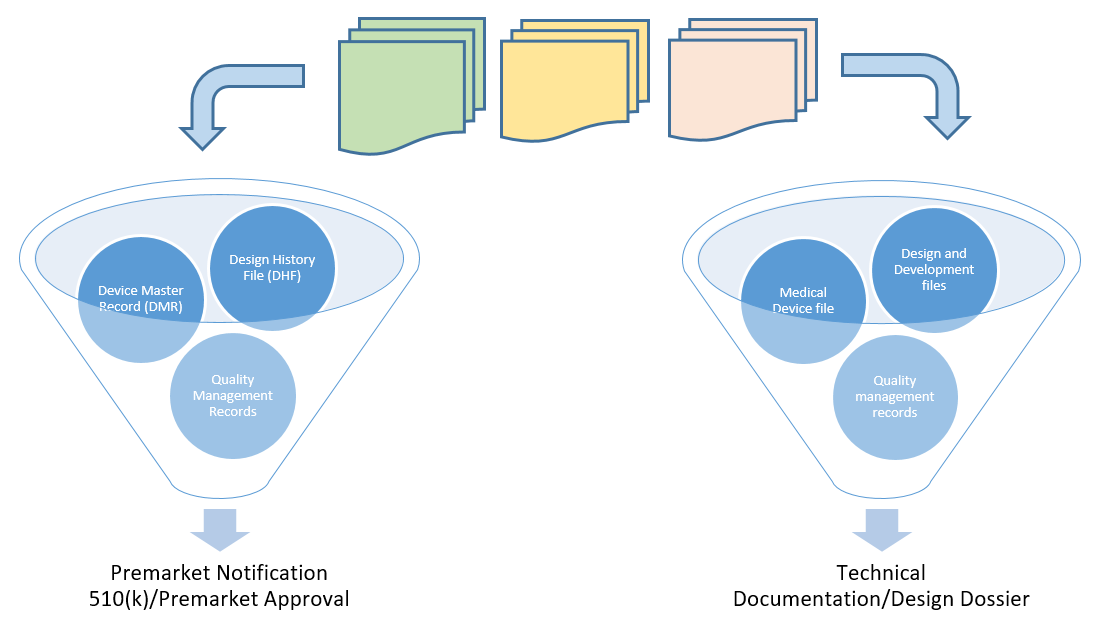
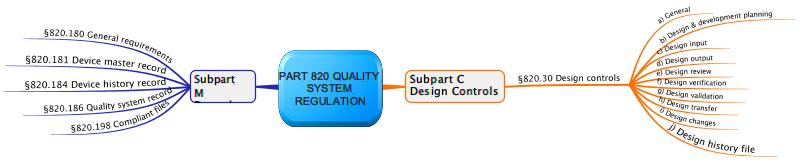
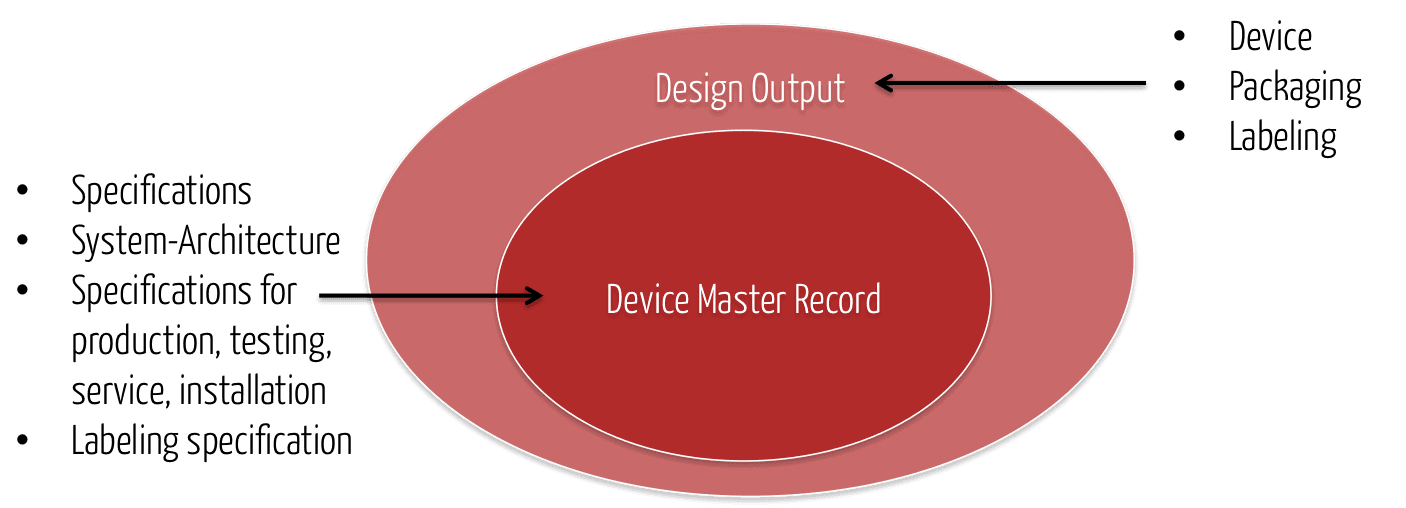
Design History Files (DHF), Device Master Records (DMR), Device History Records (DHR), Technical Documentation - The Requirements

Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR): What's the Difference?

Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR): What's the Difference?

Design History File (DHF), the Device Master Record (DMR) and the Device History Record (DHR) - YouTube

-Tech%20File.png)