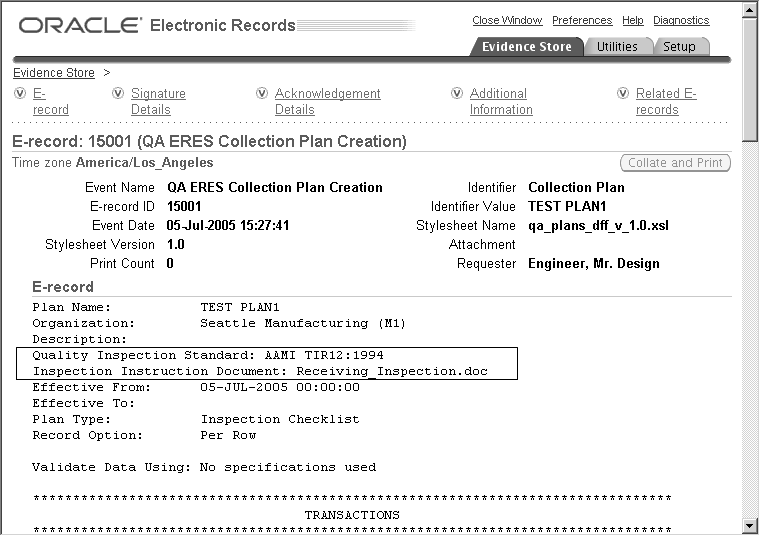
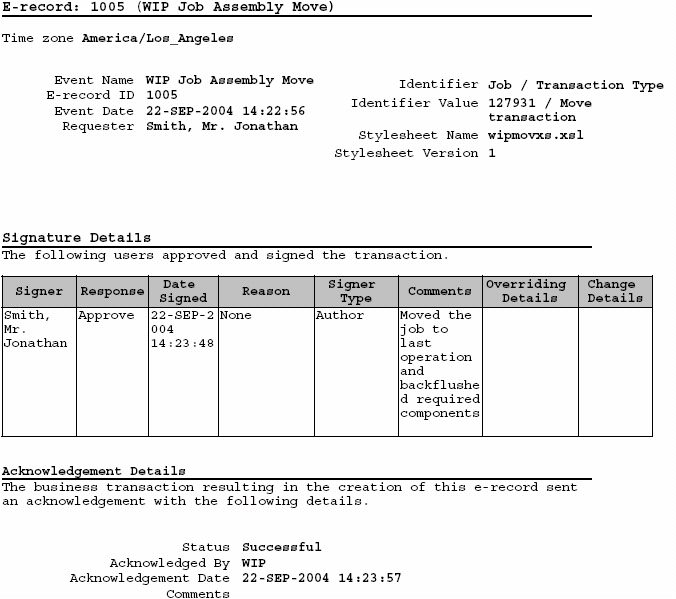
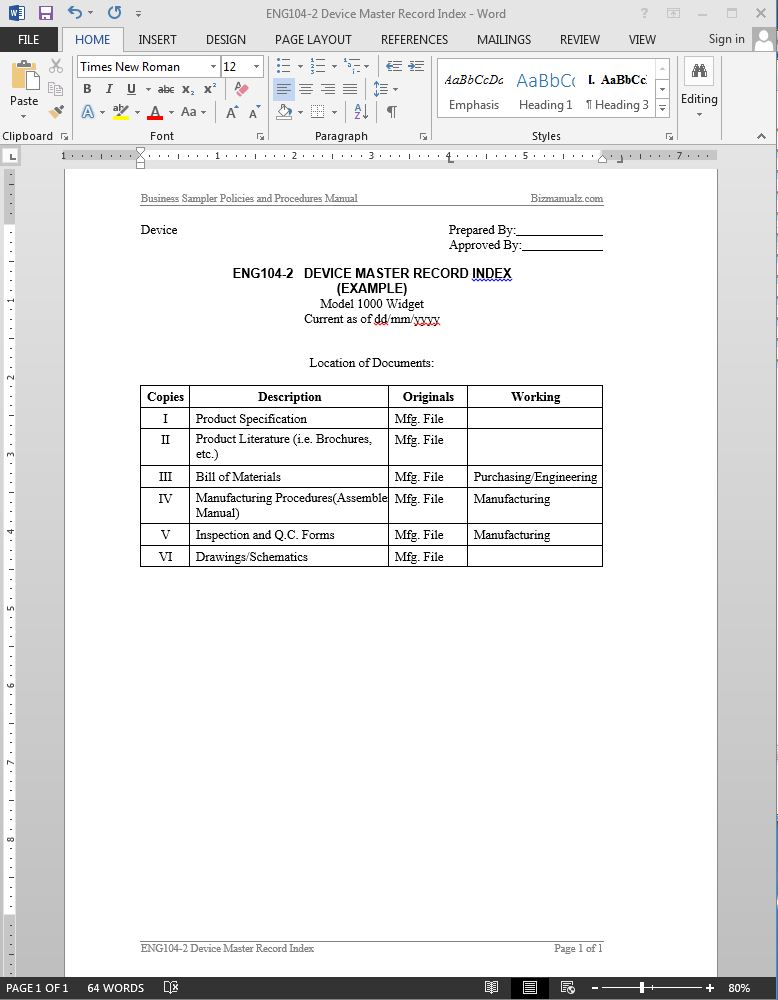
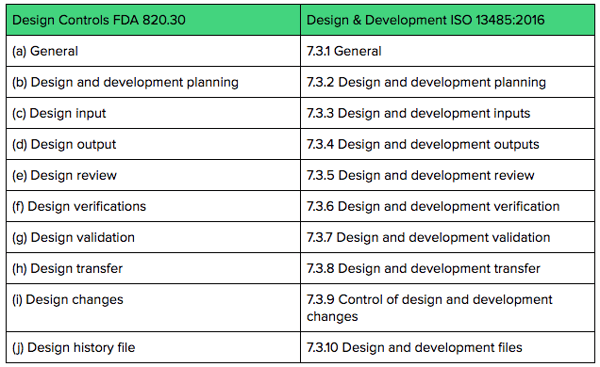
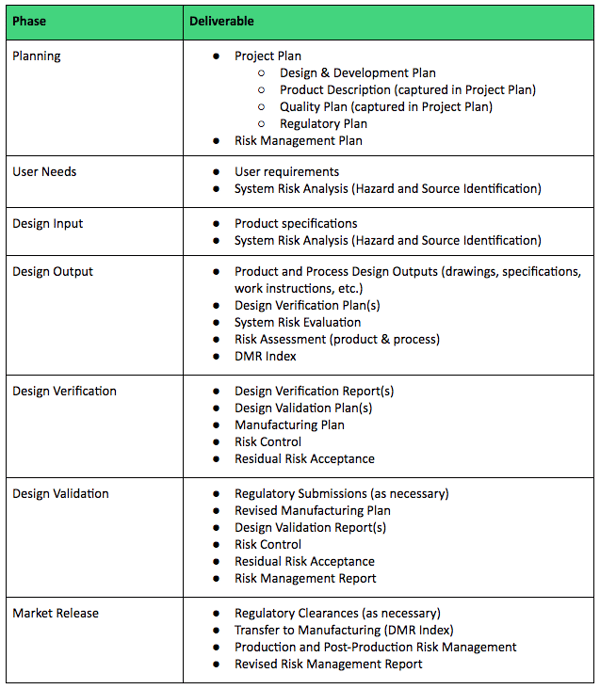
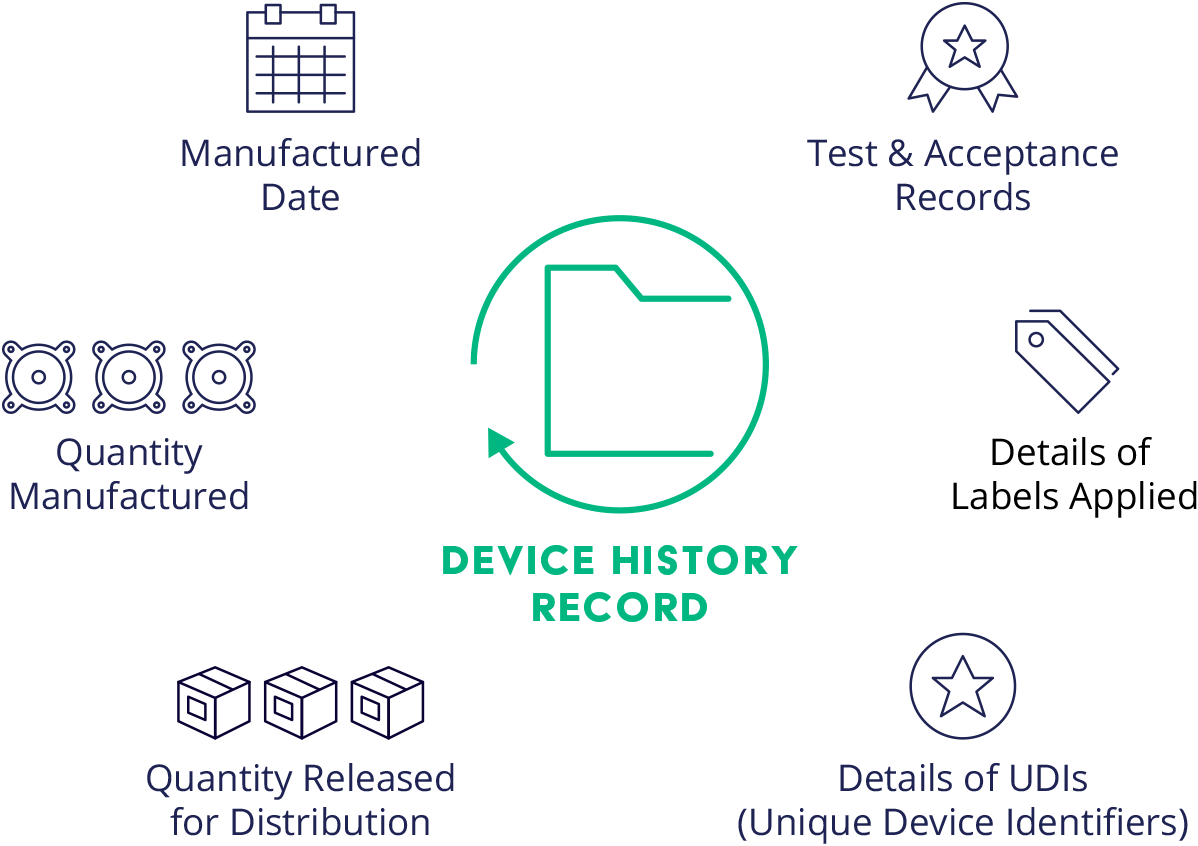
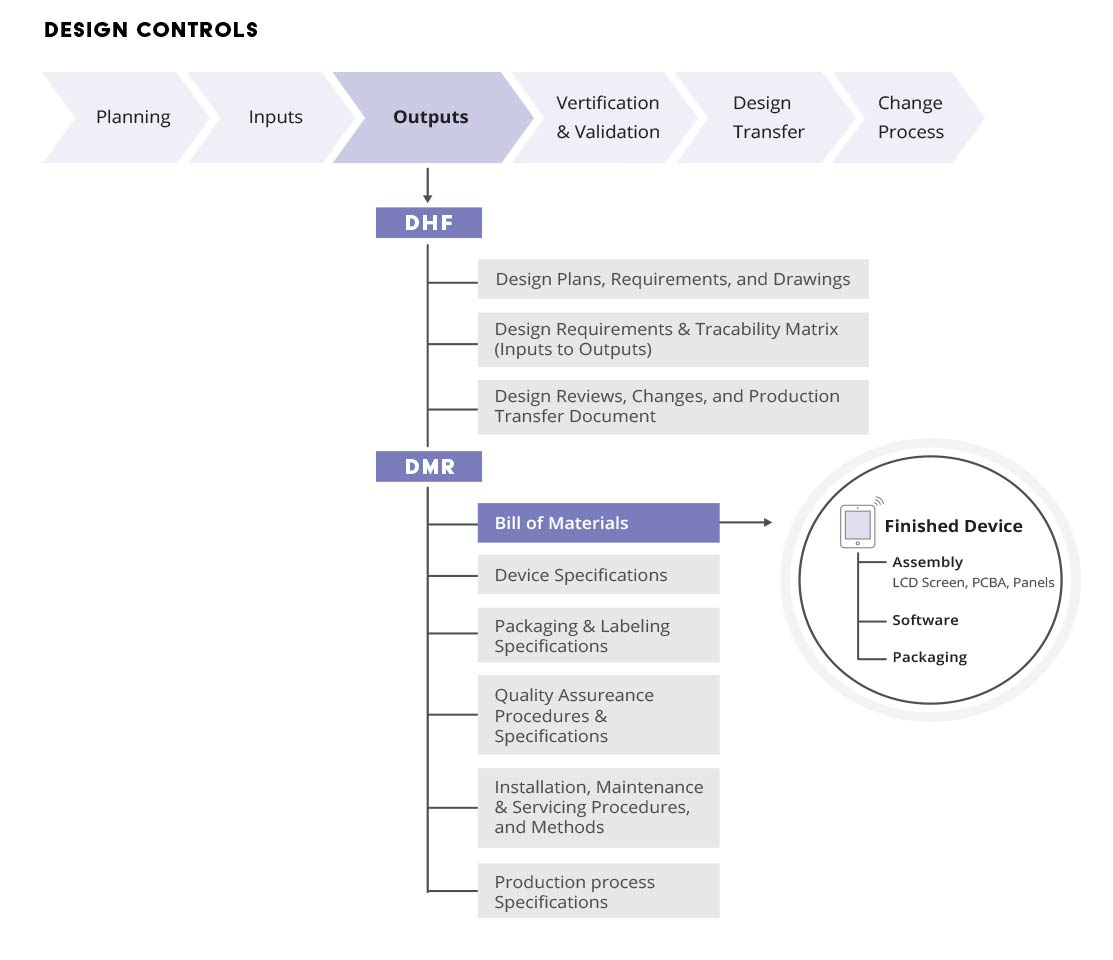
Design History File DHF, Device Master Record DMR, Device History Record DHR and Technical File TF - YouTube
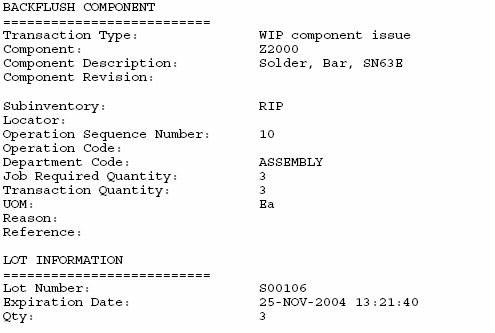
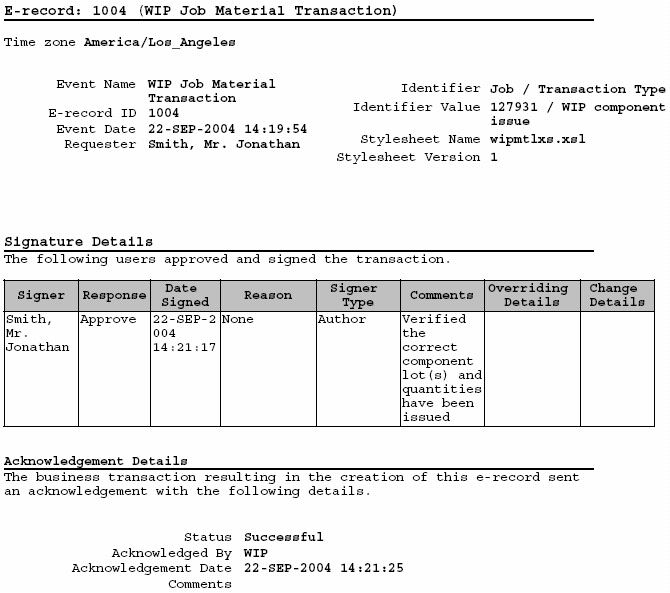
OBSERVATION 1 The device history record does not demonstrate that the device was manufactured in accordance with the device ma

Design History File (DHF), the Device Master Record (DMR) and the Device History Record (DHR) - YouTube