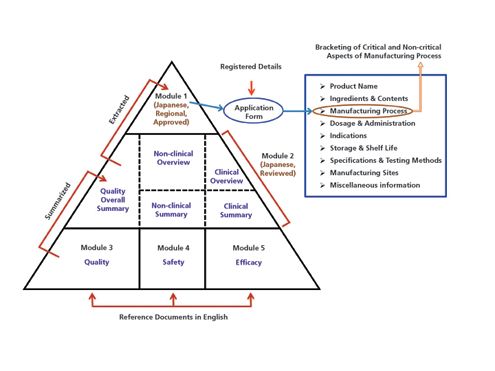
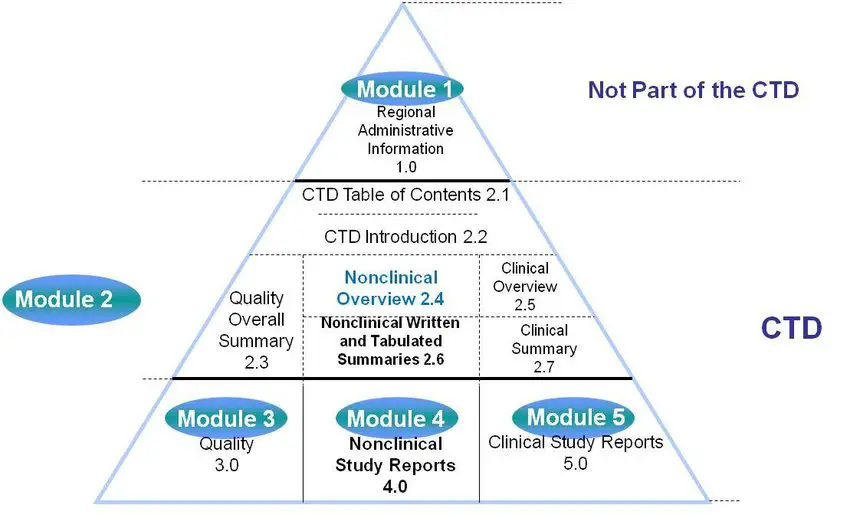
Drug substances in the drug product dossier - - Quality documentation requirements for marketing authorizations of medicinal products in Europe
eCTD - Neue Wege der elektronischen Einreichung - Vernetzung elektronischer regulatorischer Prozesse -
WHO Guidelines on submission of documentation for the pilot procedure for prequalification of similar biotherapeutic products fo