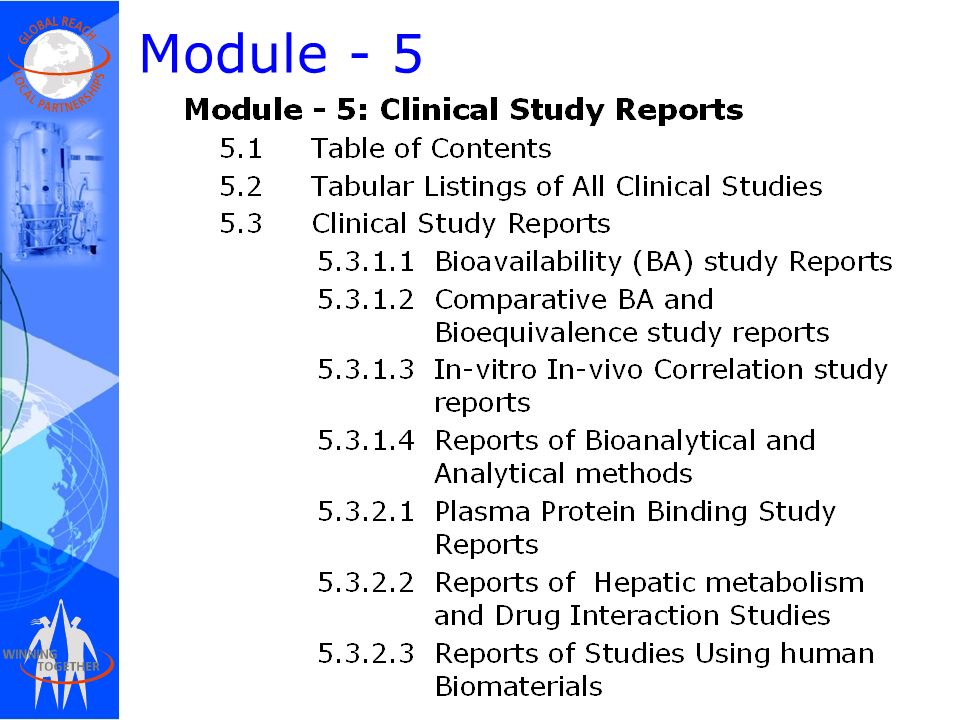
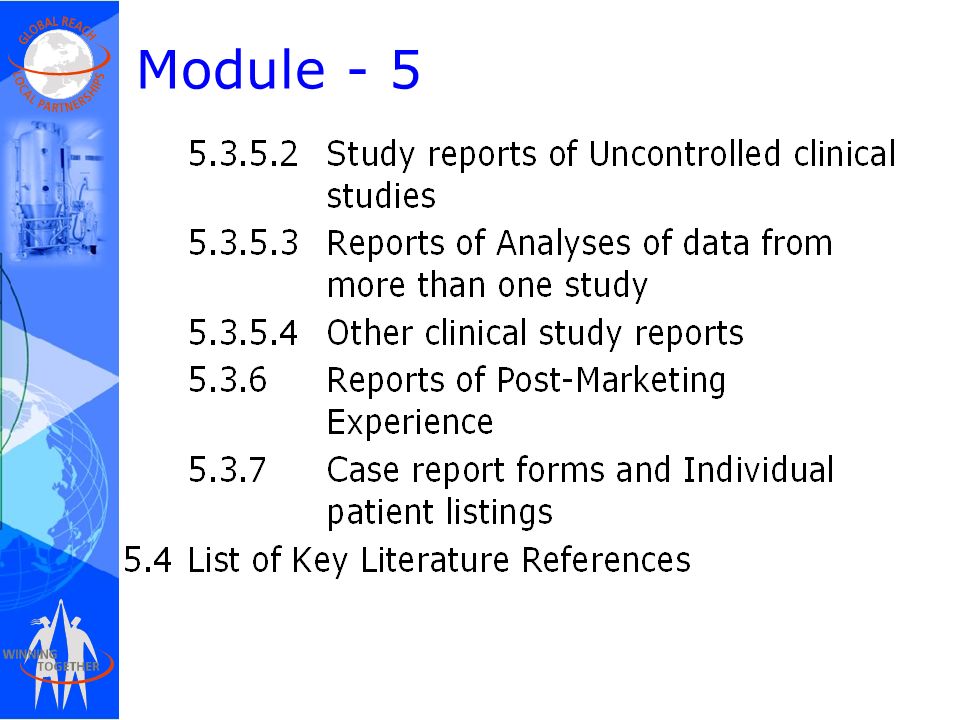
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience
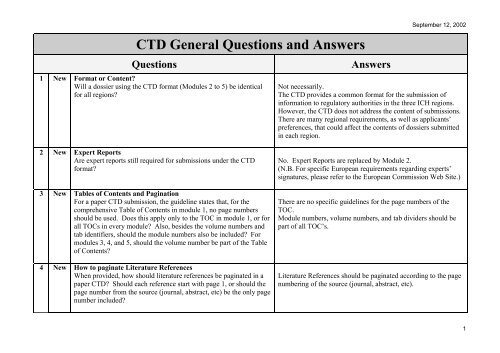
Practical Guidance For the Paper Submission of Regulatory Information in Support of a Marketing Authorisation Application When U
A Study of procedures for Dossier Preparation and their marketing authorisation in different countries of selected drug(s)

ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub

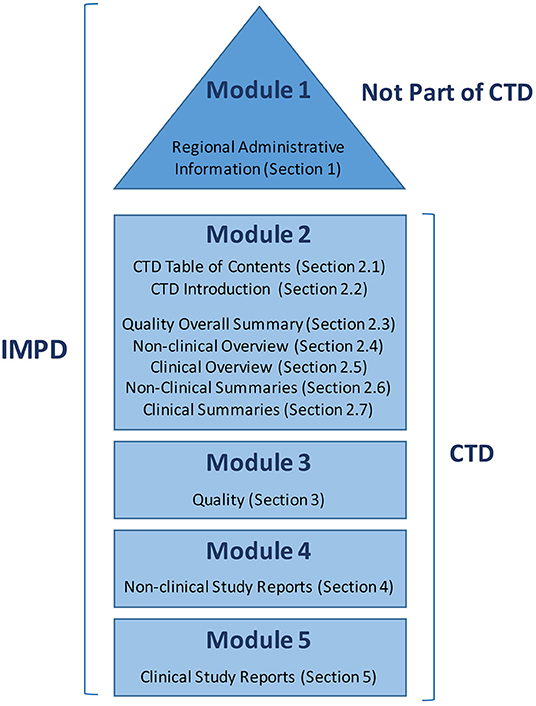
Schematic representation of the five modules in the Common Technical... | Download Scientific Diagram

Representation of the components of the CTD. The nonclinical components... | Download Scientific Diagram