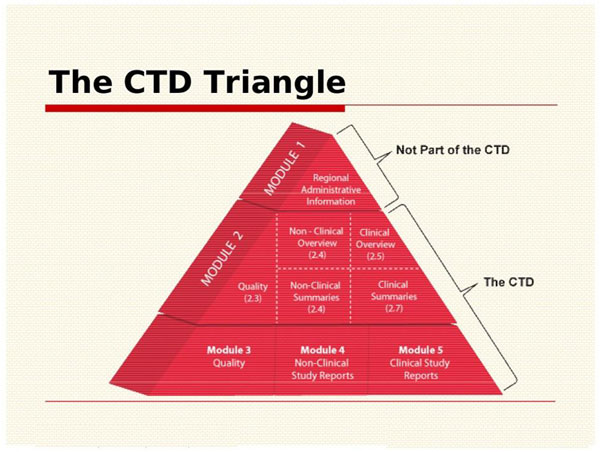
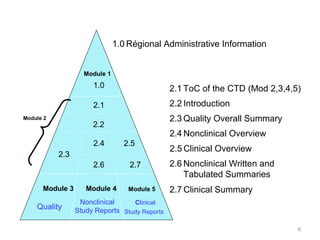
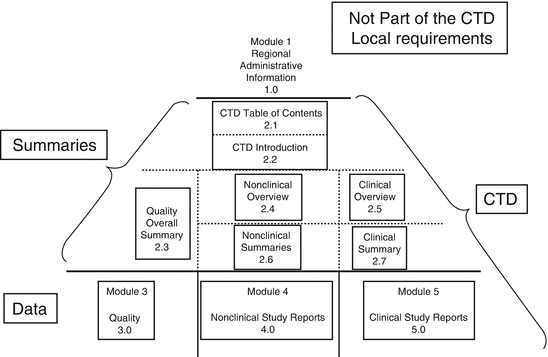
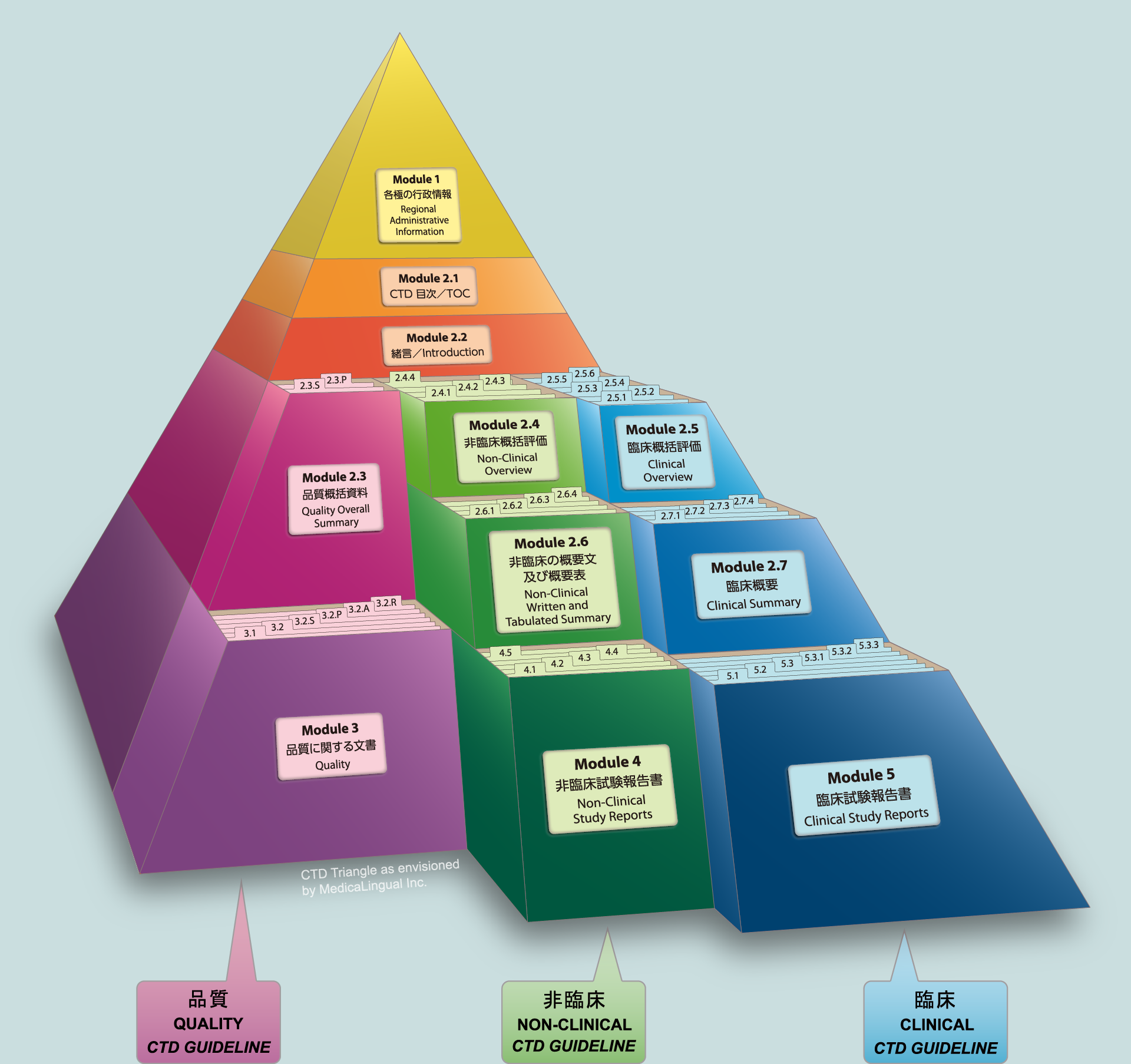
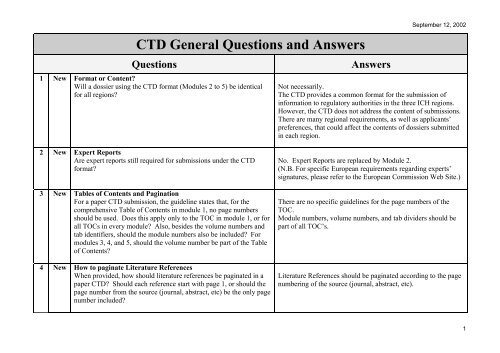
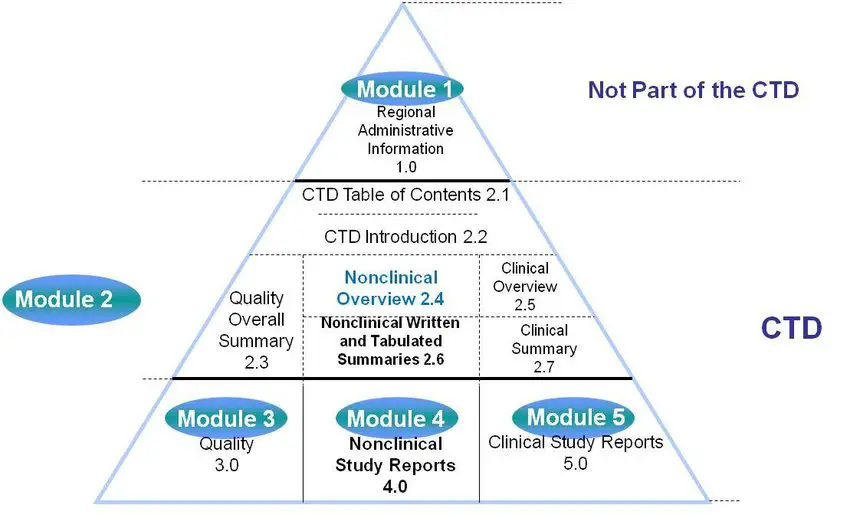
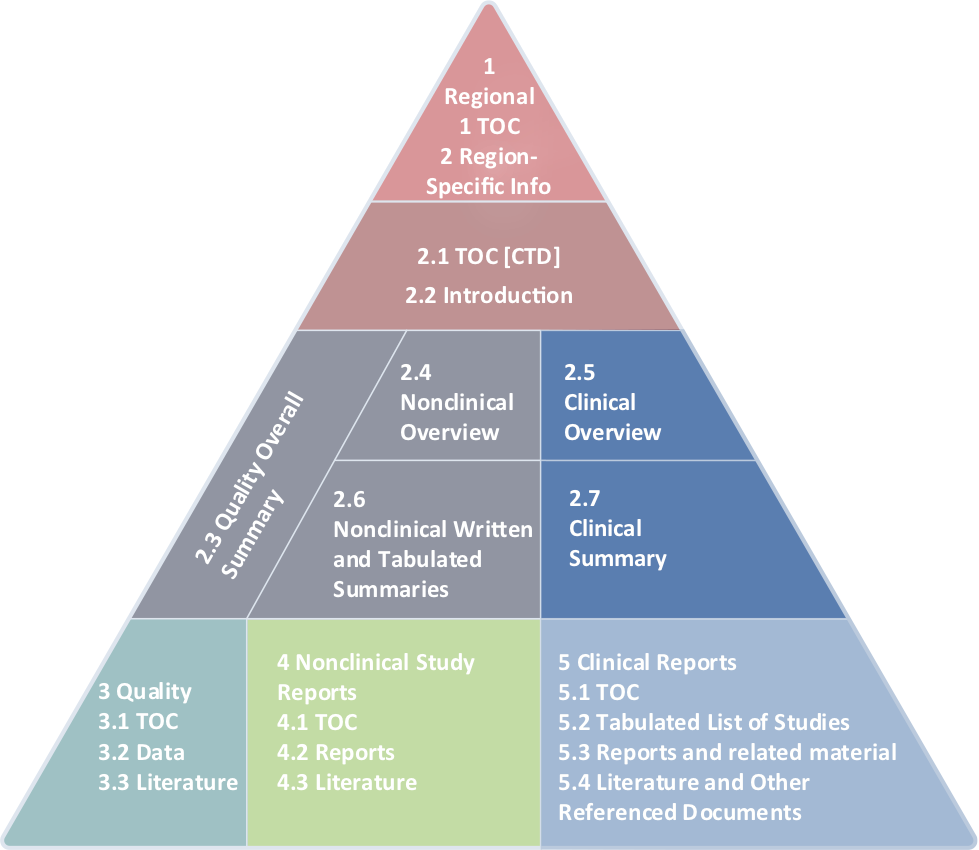
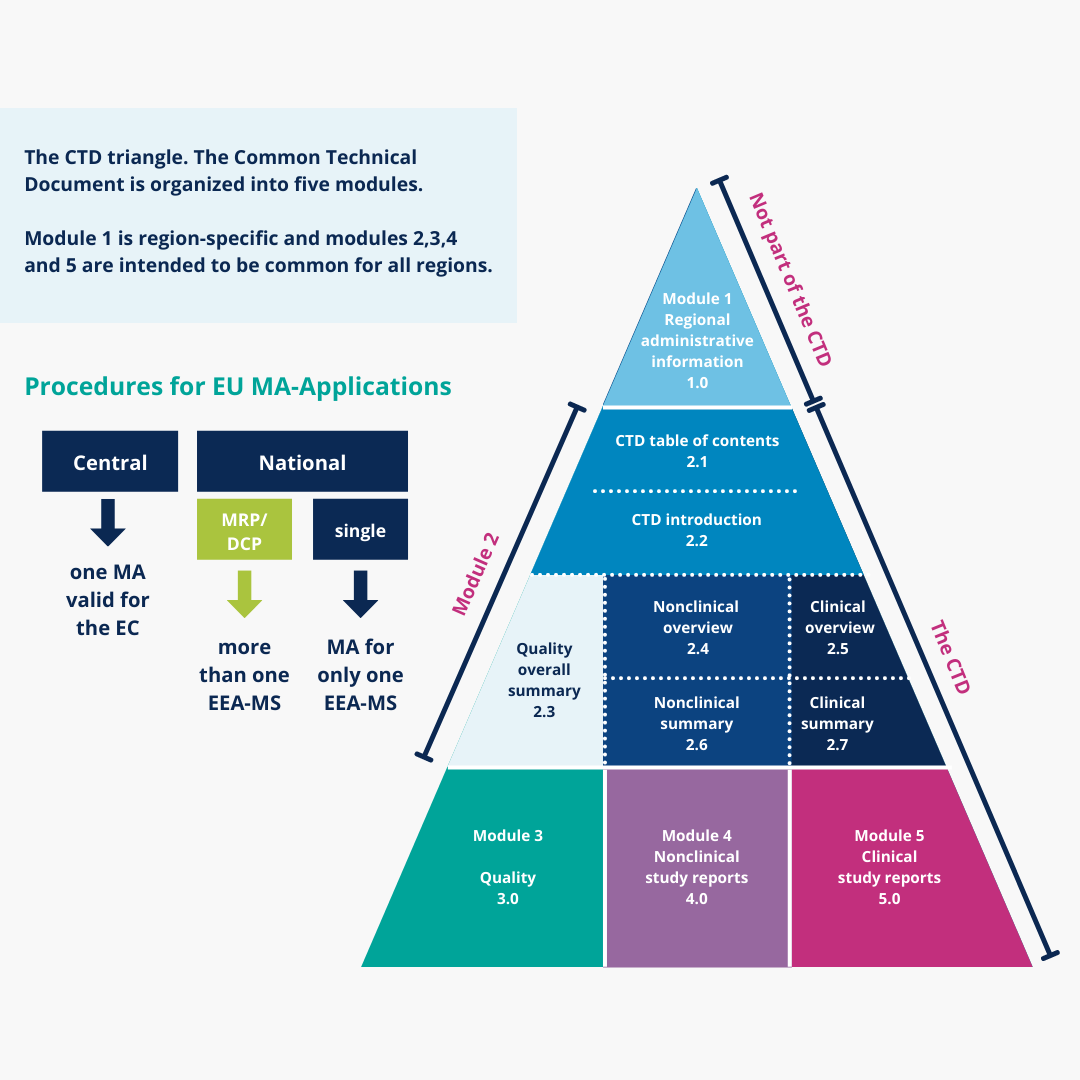
ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub
![PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/30903f1fc51c4917a2877b9cf3756ccc7fc6425a/16-Table2-1.png)
PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar

1 COMMON TECHNICAL DOCUMENT / ORIGIN OF CTD… ICH EWG CTD WAS OFFICIALLY SIGNED OFF IN NOVEMBER 2000, AT 5 TH ICH CONFERENCE; SAN DIEGO,CALIFORNIA. - ppt download
Potential to Simplify the Writing of Submission Documents: Evaluation of Publicly Available Module 2 Documents in Drug Submissio
Exposé zum Thema: eCTD - Neue Wege der elektronischen Arzneimittelzulassung und die Vernetzung elektronischer regulatorischer P

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

TuraSkills shares tip for writing #Module 2.5 #Section 2.5.2 #Overview of Biopharmaceutics #Clinical overview #… | Writing tips, Marketing data, Clinical chemistry
eCTD - Neue Wege der elektronischen Einreichung - Vernetzung elektronischer regulatorischer Prozesse -

Schematic representation of the five modules in the Common Technical... | Download Scientific Diagram