Drug substances in the drug product dossier - - Quality documentation requirements for marketing authorizations of medicinal products in Europe
WHO Guidelines on submission of documentation for the pilot procedure for prequalification of similar biotherapeutic products fo

Representation of the components of the CTD. The nonclinical components... | Download Scientific Diagram

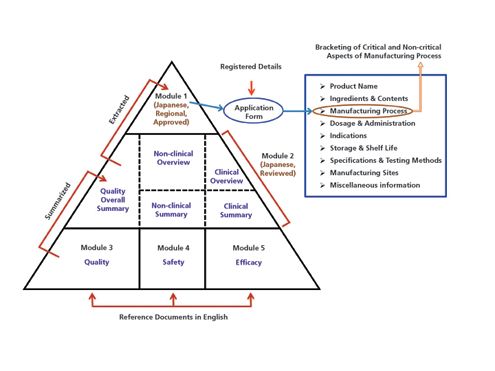
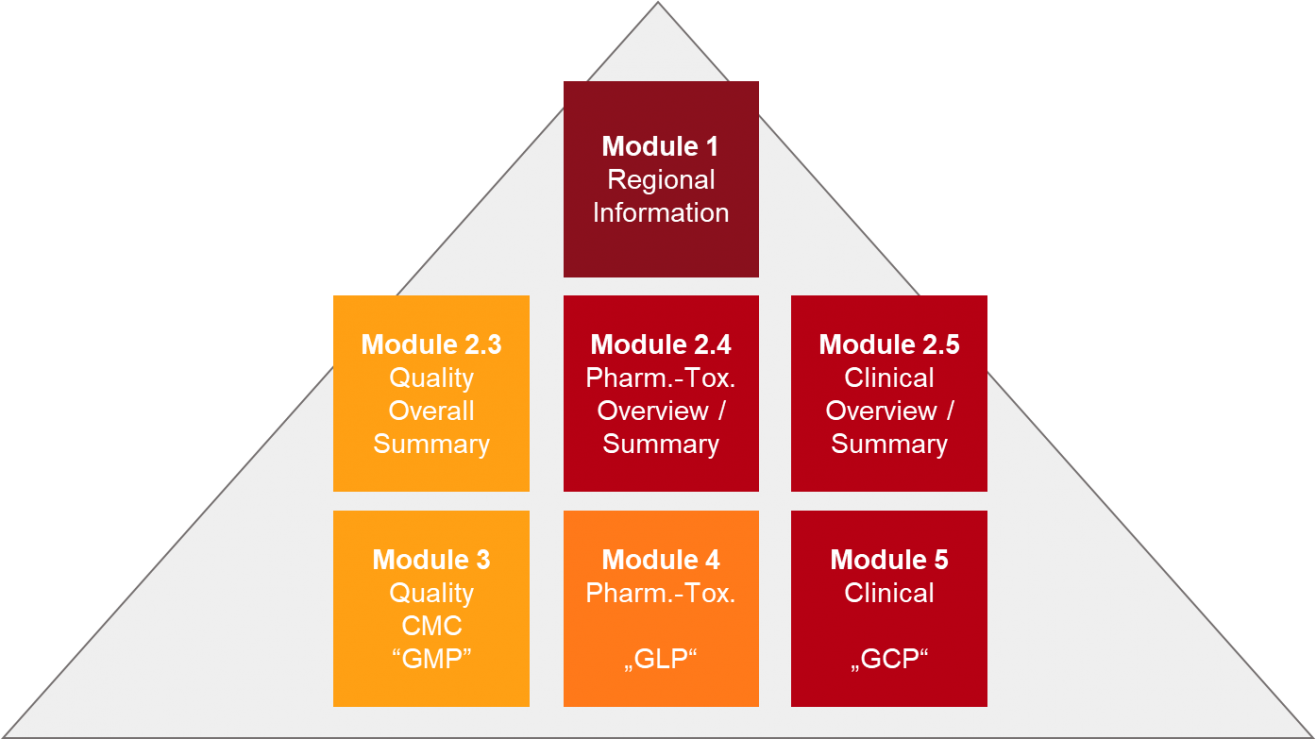
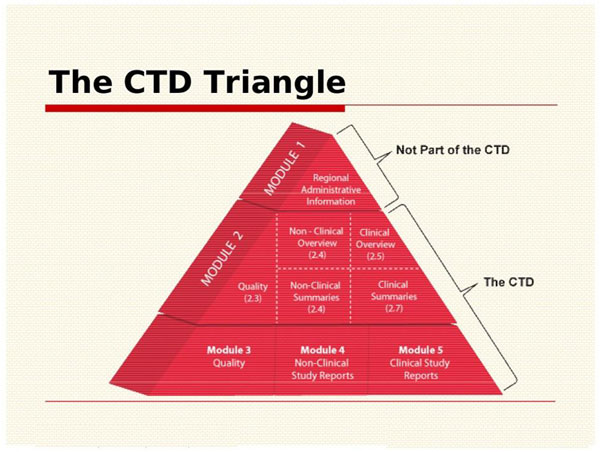
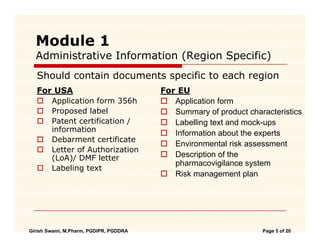
ClinProve - CTD Structure The Common Technical Document is divided into five modules: 1. Administrative and prescribing information 2. Overview and summary of modules 3 to 5 3. Quality (pharmaceutical documentation) 4.
eCTD - Neue Wege der elektronischen Einreichung - Vernetzung elektronischer regulatorischer Prozesse -
![PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/30903f1fc51c4917a2877b9cf3756ccc7fc6425a/16-Table2-1.png)