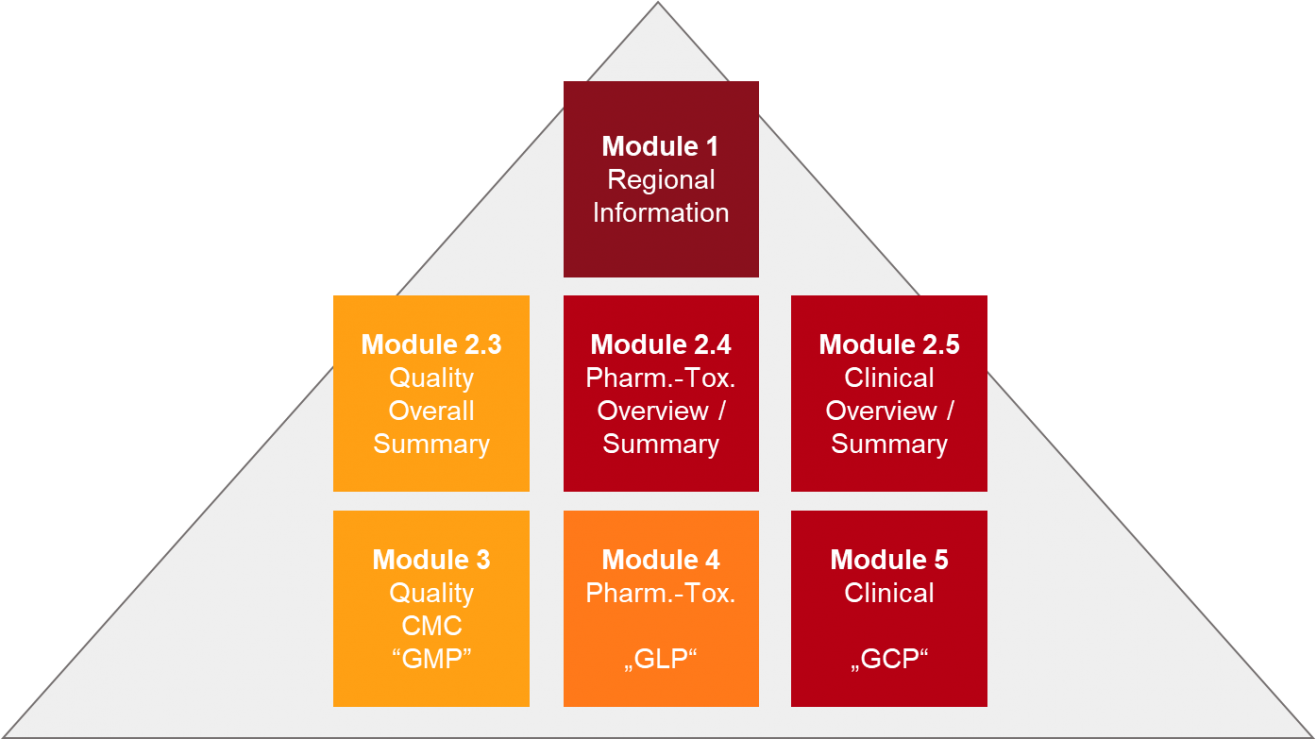
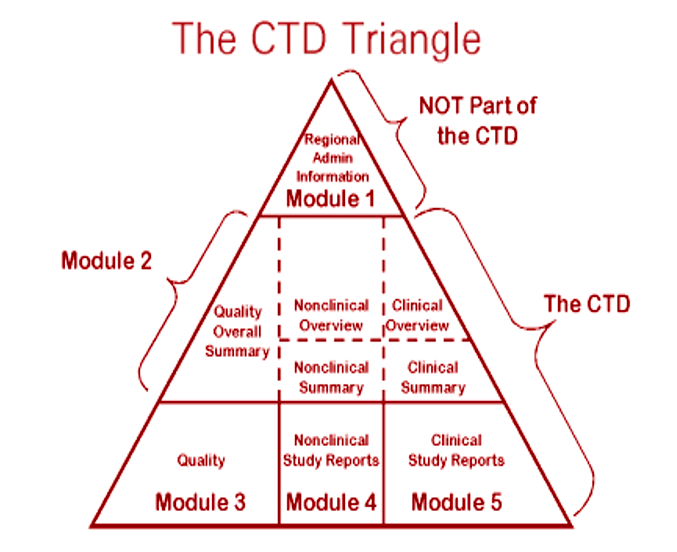
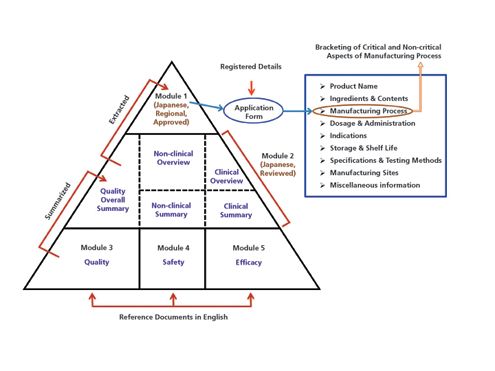
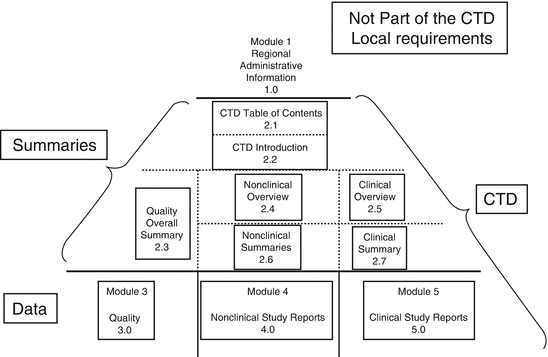
The Common Technical Document Elements (Source: ?Implementation of the... | Download Scientific Diagram

Nonclinical Information in the Common Technical Document: Opportunities for Content Reuse Peggy Zorn, MPI Research Susan Mattano, Pfizer, Inc. - ppt download
ELECTRONIC COMMON TECHNICAL DOCUMENT (eCTD): A REVIEW OF HISTORY, BENEFITS OF IMPLEMENTING, CHALLENGES, MODULES, RISKS INVOLVED



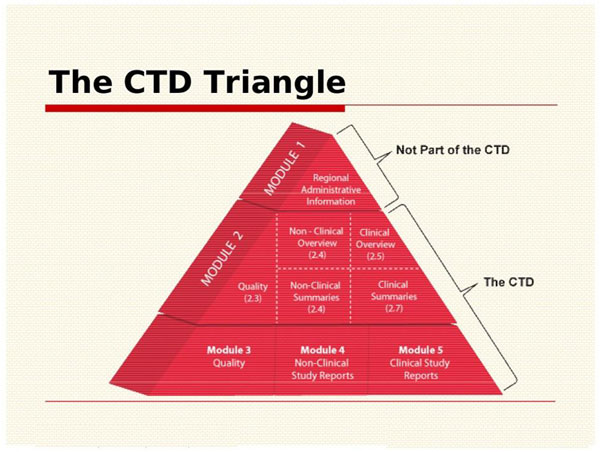
(1).png)