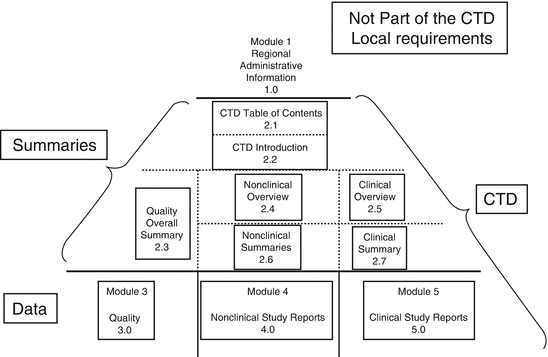
The Common Technical Document Elements (Source: ?Implementation of the... | Download Scientific Diagram
Annex 4] Organization of the Common Technical Document For the Registration of Pharmaceuticals for Human Use (With reference t
Potential to Simplify the Writing of Submission Documents: Evaluation of Publicly Available Module 2 Documents in Drug Submissio



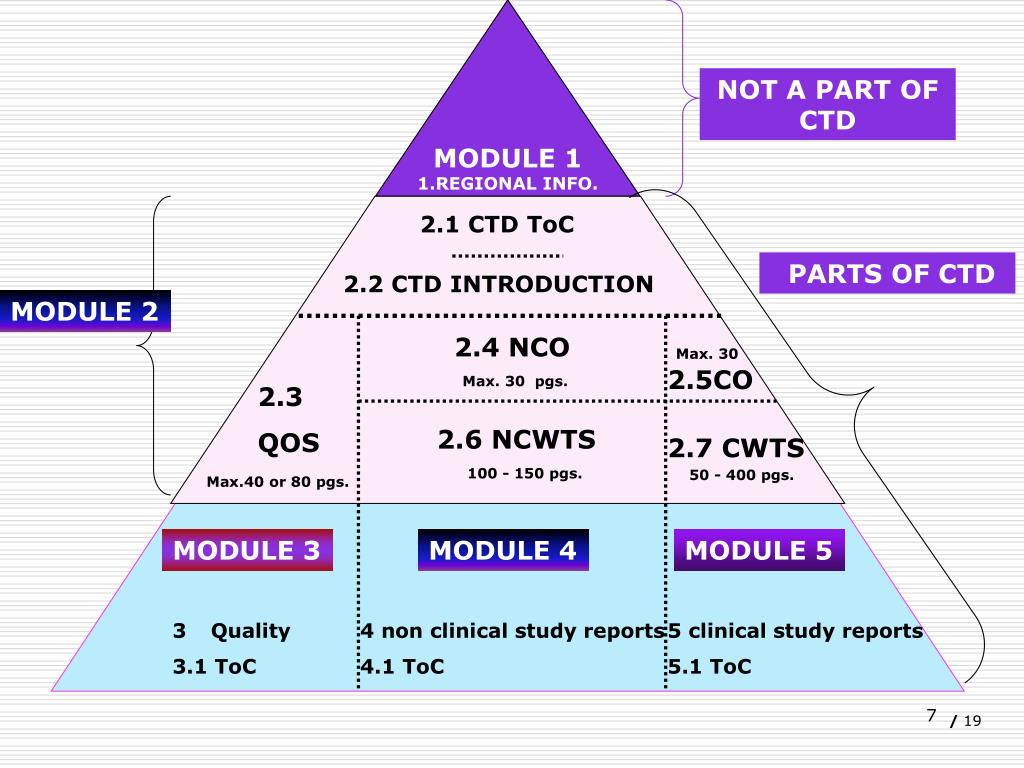
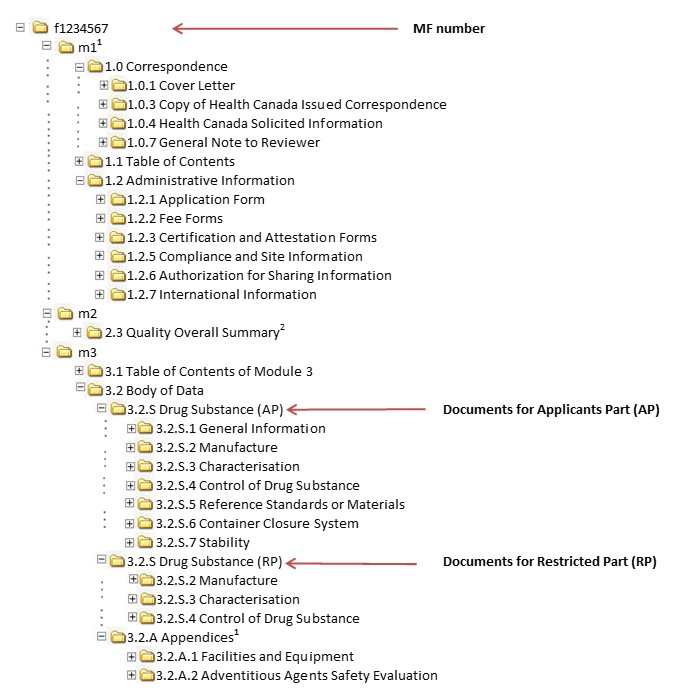







![Common technical document triangle [7] | Download Scientific Diagram Common technical document triangle [7] | Download Scientific Diagram](https://www.researchgate.net/publication/320372658/figure/fig1/AS:550839028203520@1508341666076/Common-technical-document-triangle-7.png)