
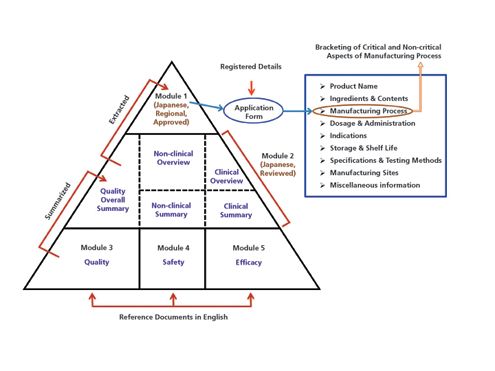
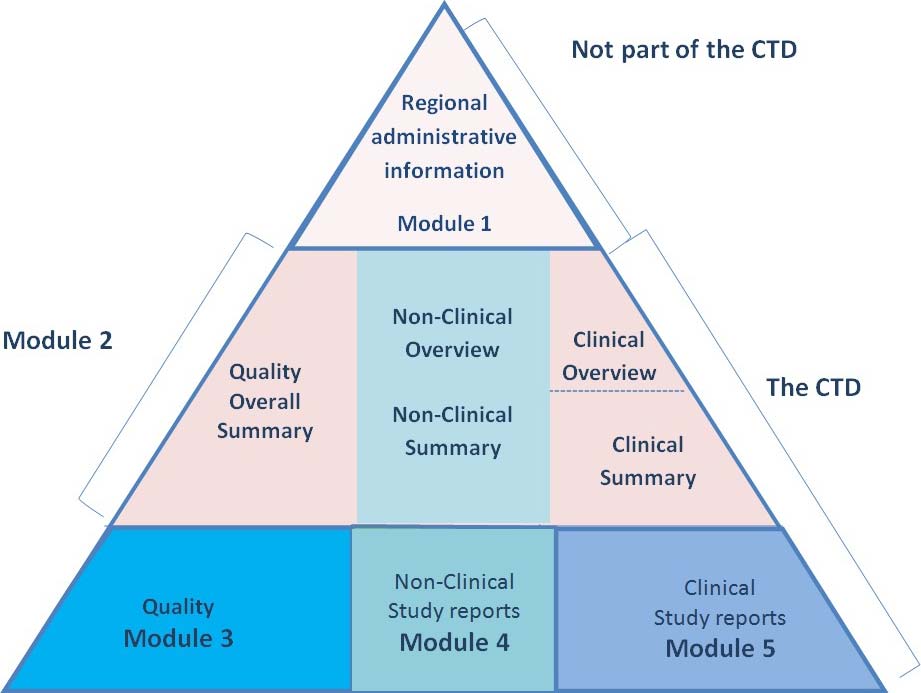
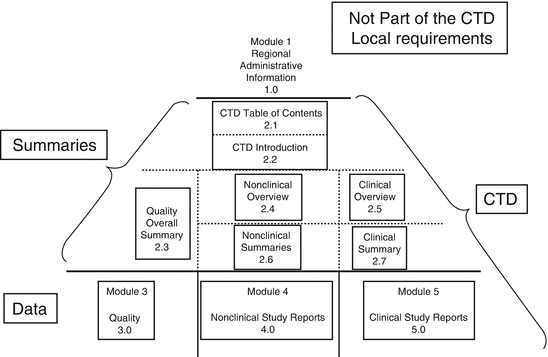
ClinProve - CTD Structure The Common Technical Document is divided into five modules: 1. Administrative and prescribing information 2. Overview and summary of modules 3 to 5 3. Quality (pharmaceutical documentation) 4.

Common Technical Document (CTD/ACTD/EAC/GCC/JORDAN/IRAN etc.) in TUGHLAKABAD EXTN.,, New Delhi, Medwisdom Lifesciences Private Limited | ID: 23884030697
ELECTRONIC COMMON TECHNICAL DOCUMENT (eCTD): A REVIEW OF HISTORY, BENEFITS OF IMPLEMENTING, CHALLENGES, MODULES, RISKS INVOLVED

ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub

The Common Technical Document Elements (Source: ?Implementation of the... | Download Scientific Diagram

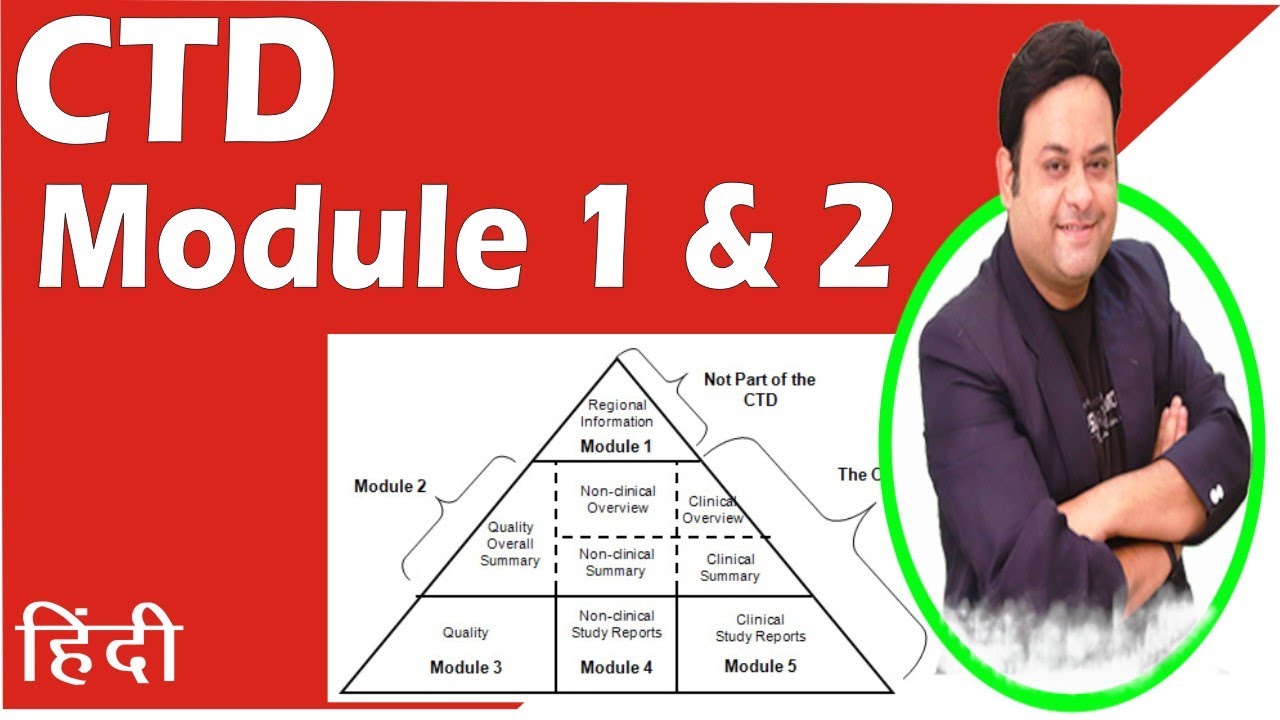
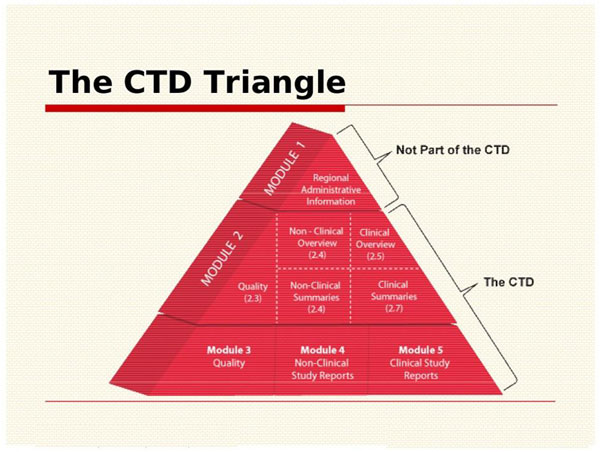

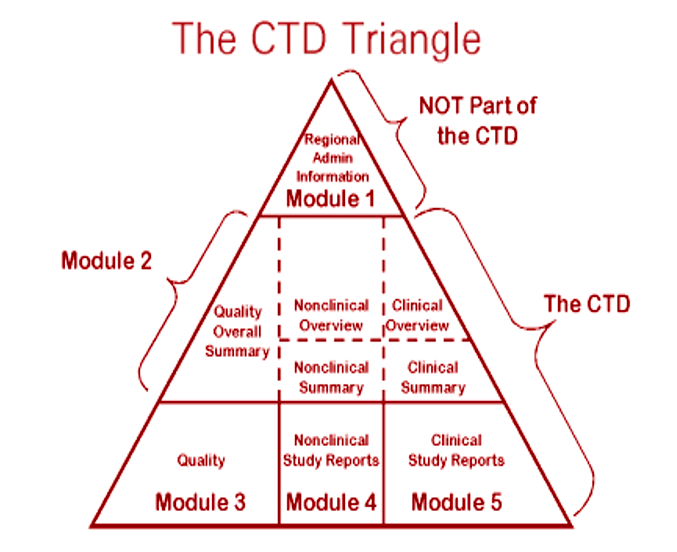









(1).png)
