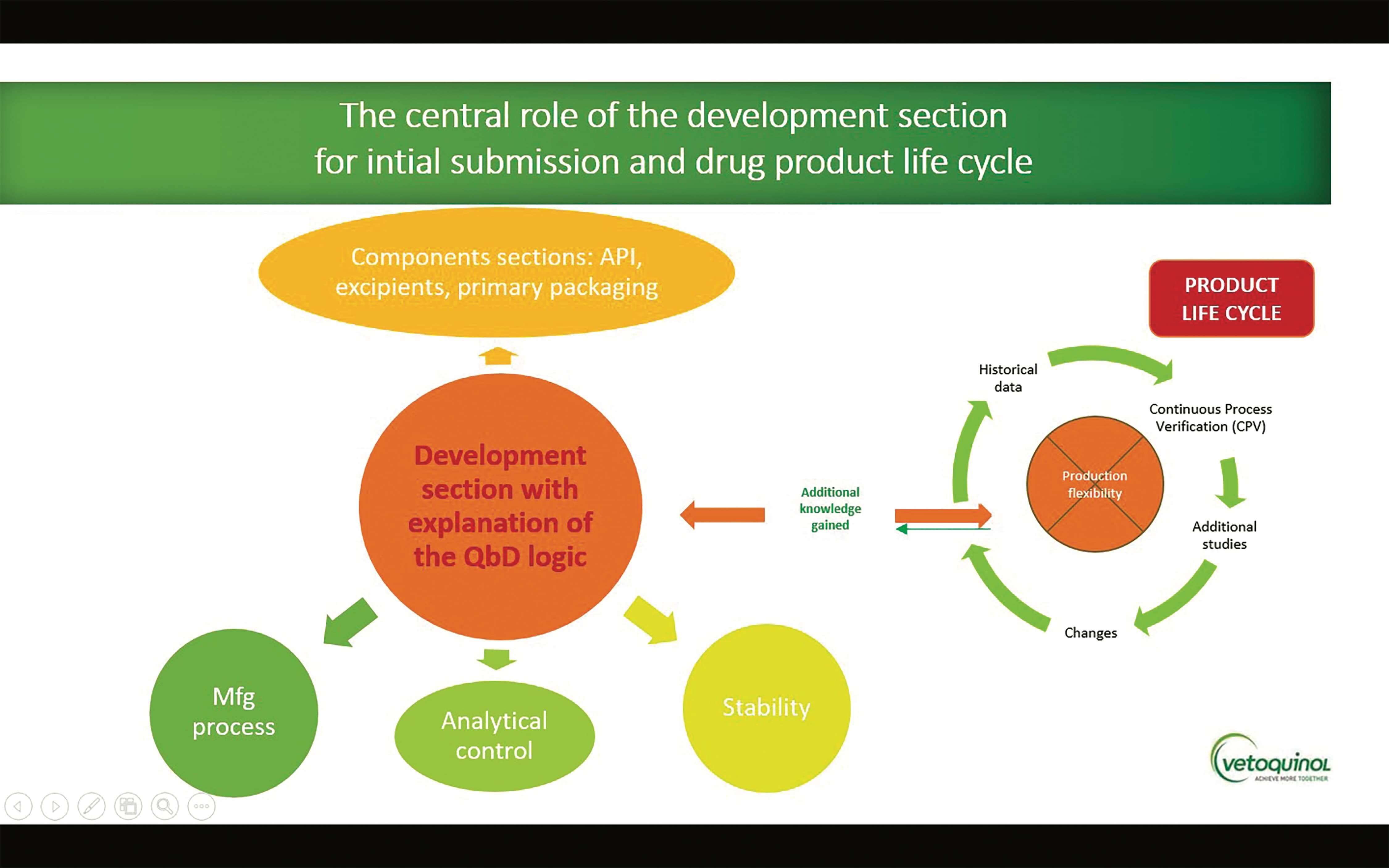
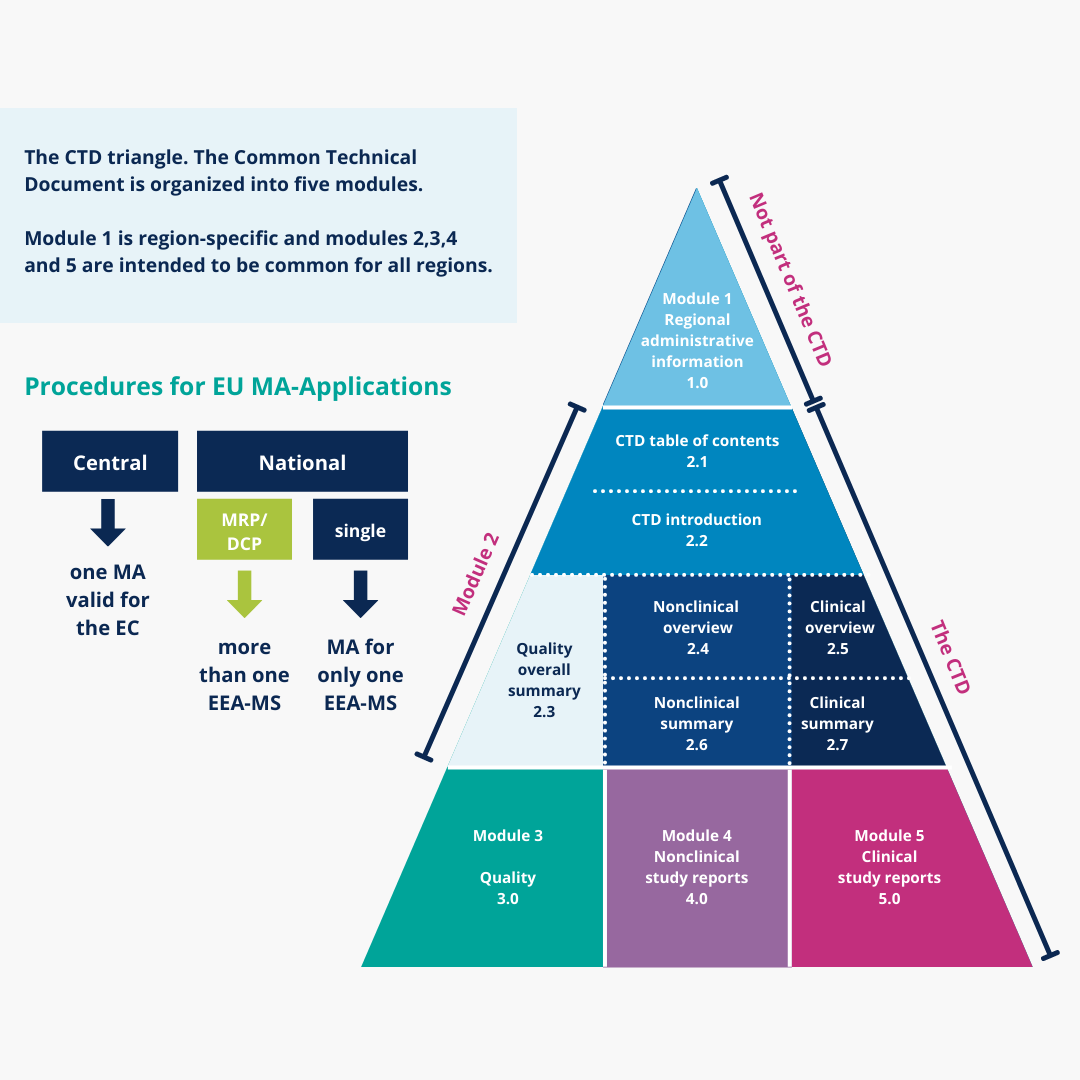
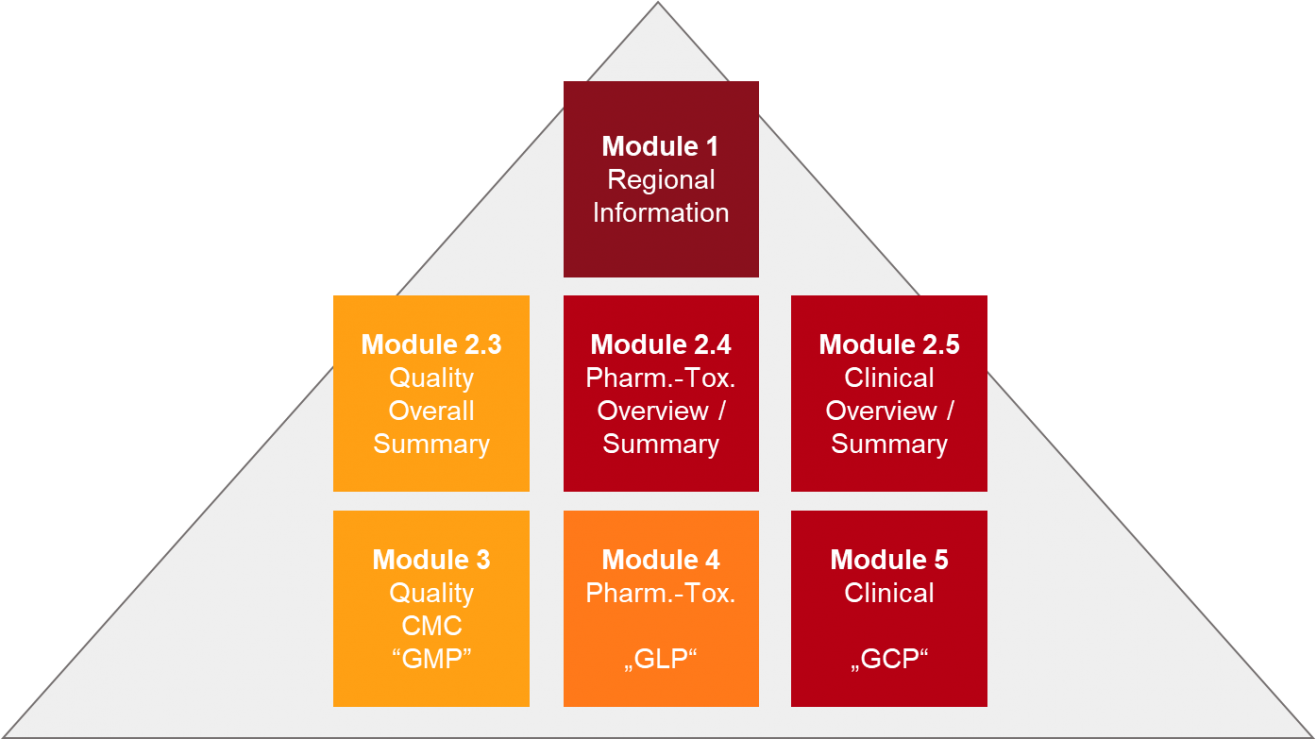
Change Happens: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (CMC Forum) - BioProcess InternationalBioProcess International
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan

Transitioning Chemistry, Manufacturing, and Controls Content With a Structured Data Management Solution: Streamlining Regulatory Submissions - Journal of Pharmaceutical Sciences

The Future of CMC Regulatory Submissions: Streamlining Activities Using Structured Content and Data Management - ScienceDirect

ICH CTD QUALITY Part -CMC Module 3 Drug Substance Video by Rajashri Ojha at Raaj PharmaeLearning - YouTube

PDF) PREPARATION AND REVIEW OF CHEMISTRY, MANUFACTURING AND CONTROL (CMC) SECTIONS OF CTD DOSSIER FOR MARKETING AUTHORIZATION

The Organisation for Professionals in Regulatory Affairs - TOPRA - How to effectively prepare and manage module 3 of the dossier covering pharmaceutical (CMC) information. Get up to date with the latest

The Future of CMC Regulatory Submissions: Streamlining Activities Using Structured Content and Data Management - ScienceDirect