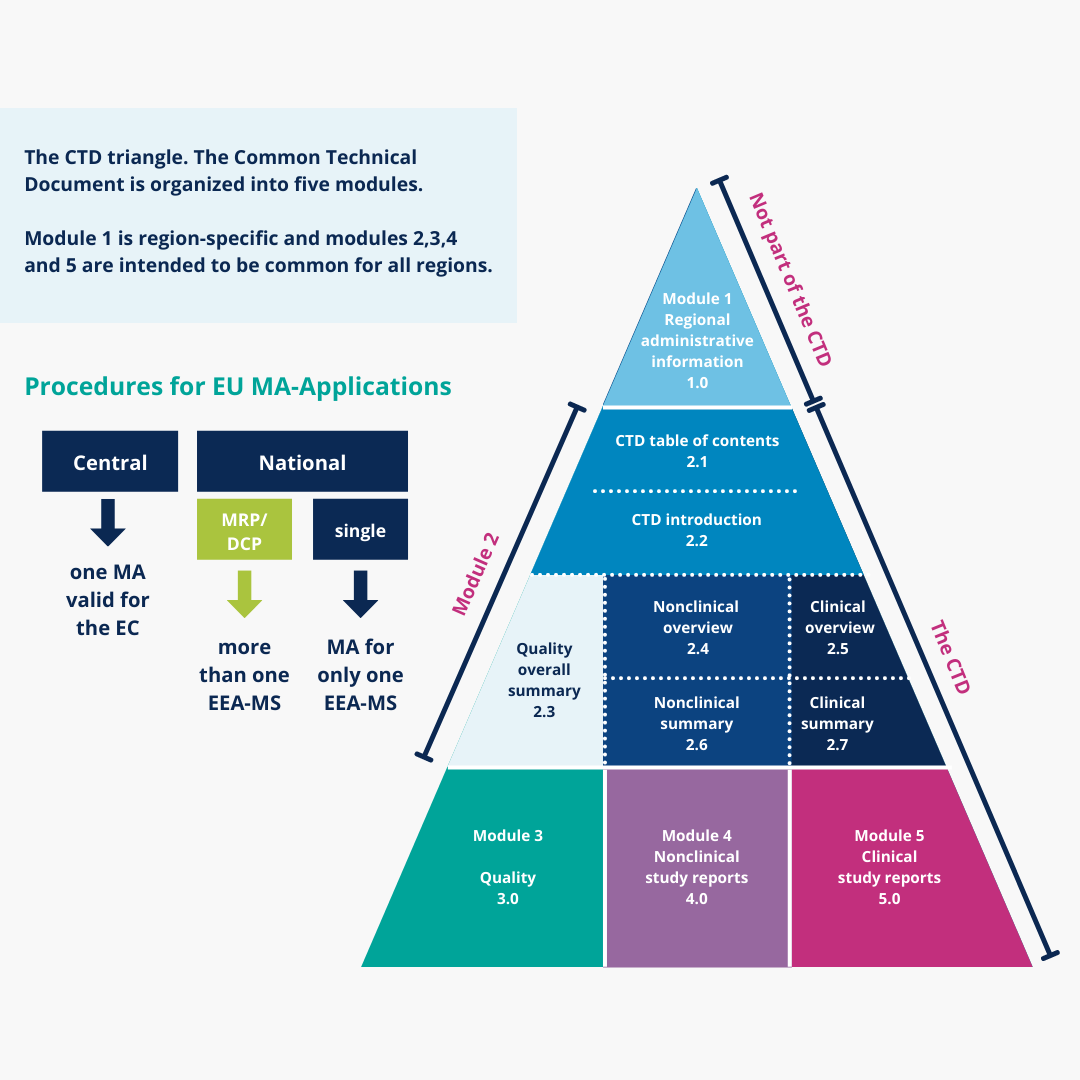
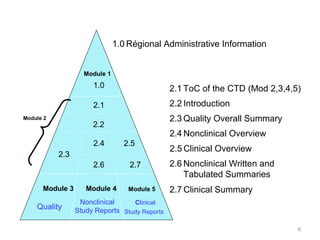
Last Update June 13 ' ToC of Module 1 or overall ToC, including Module ToC of the CTD (Mod 2,3,4,5) Module 1 Module 3Module 4Module ppt download

TuraSkills shares tip for writing #Module 2.5 #Clinical overview #CTD overview #Common Technical Documents # CTD #Regul… | Technical writing, Writing tips, Writing

Integramedix Ltd. Medical Sciences Training & Research - The Common Technical Document (CTD) هي مجموعة من المواصفات لملف لتسجيل الأدوية. تم تطوير CTD من قبل المؤتمر الدولي حول تنسيق المتطلبات التقنية لتسجيل

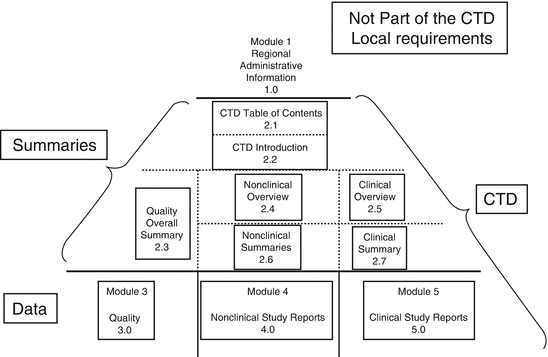
Schematic representation of the five modules in the Common Technical... | Download Scientific Diagram
Abbreviated Clinical Study Reports with Investigational Medicinal Products for Human Use: Current Guidelines and Recommendations
Preparing the Common Technical Document for Registration of Pharmaceuticals for Human Use (CTD)—Insights and Recommendations

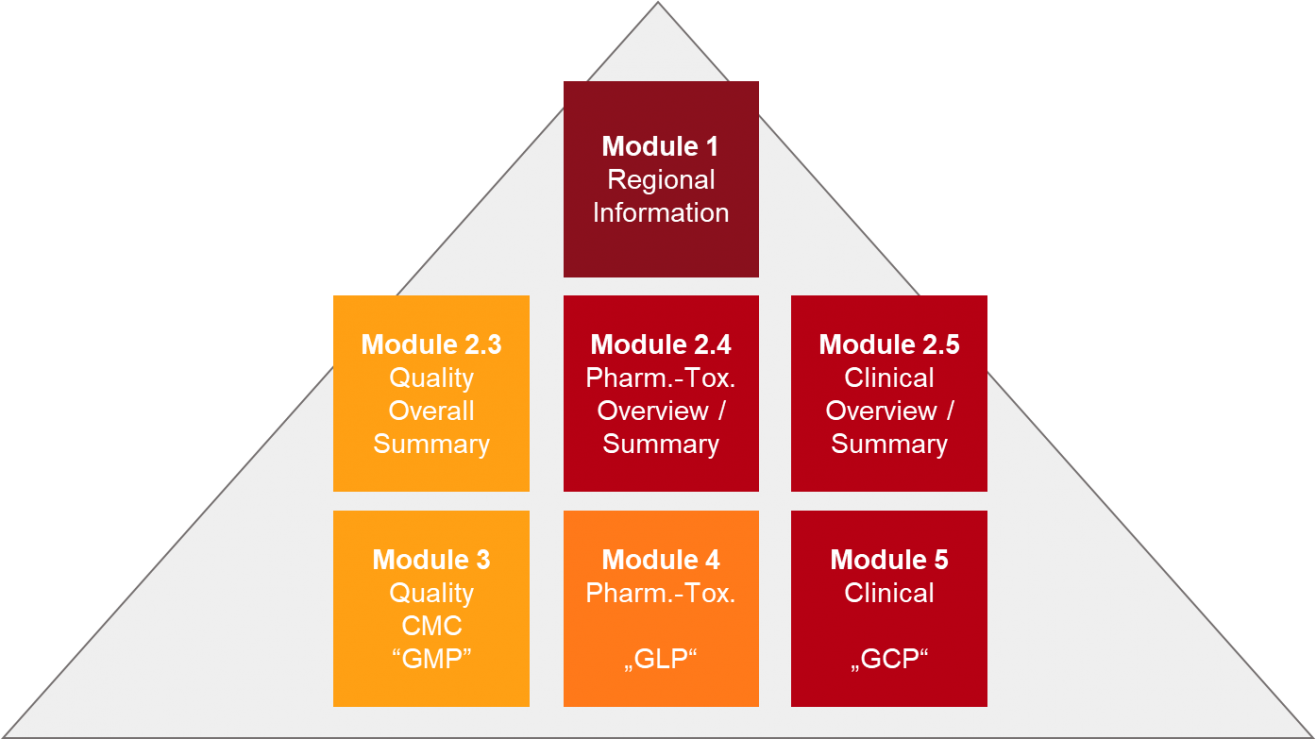
ICH M4Q Common technical document for the registration of pharmaceuticals for human use – Introduction – Orioled Hub
Progress Toward Standardization of Submissions with the Electronic Common Technical Document and the Evolving Standardization of
Medical Writer – Medical Services Interested candidates can send their resume at rchourey@cliantha.com

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH

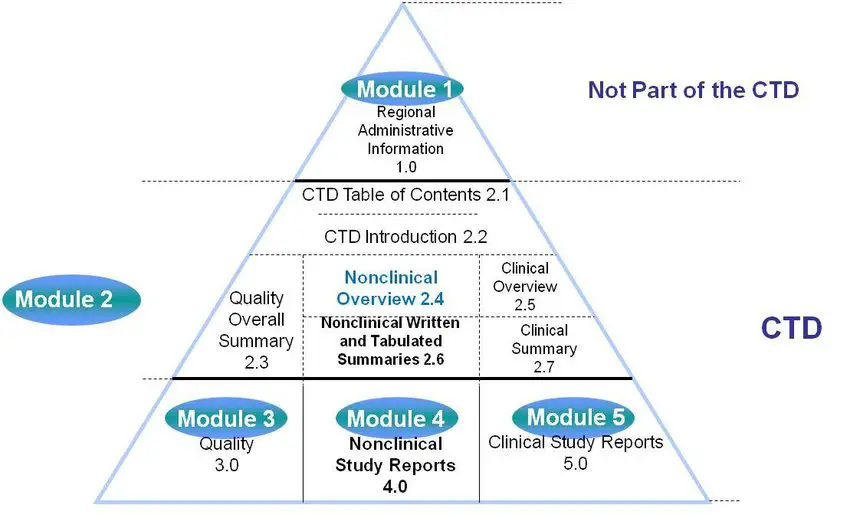
Schematic representation of the five modules in the Common Technical... | Download Scientific Diagram

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH
Potential to Simplify the Writing of Submission Documents: Evaluation of Publicly Available Module 2 Documents in Drug Submissio
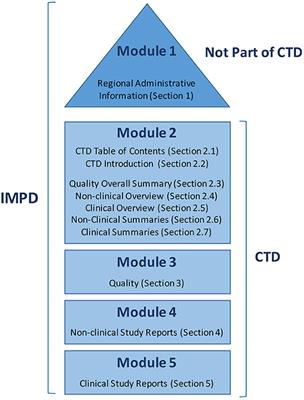
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience
M4E(R2) - Common Technical Document for the Registration of Pharmaceuticals for Human Use - Efficacy