Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales - The Lancet

Covid-19 vaccine booster shots to be offered to Americans beginning September 20, health officials say | CNN
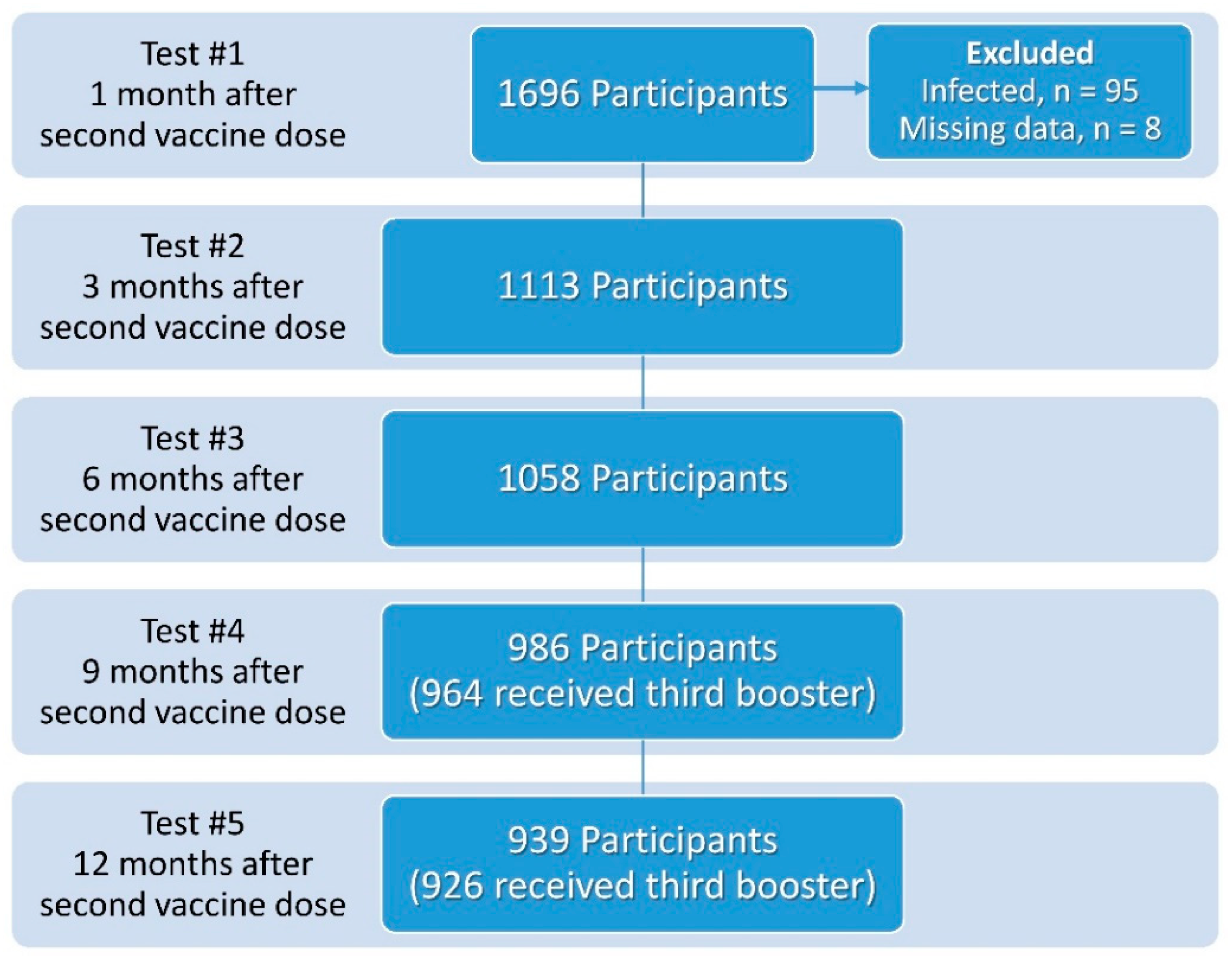
Vaccines | Free Full-Text | Third BNT162b2 Vaccine Booster Dose against SARS-CoV-2-Induced Antibody Response among Healthcare Workers

COVID-19 Vaccine Boosters vs. Third Doses: Frequently Asked Questions - Anne Arundel County Department of Health
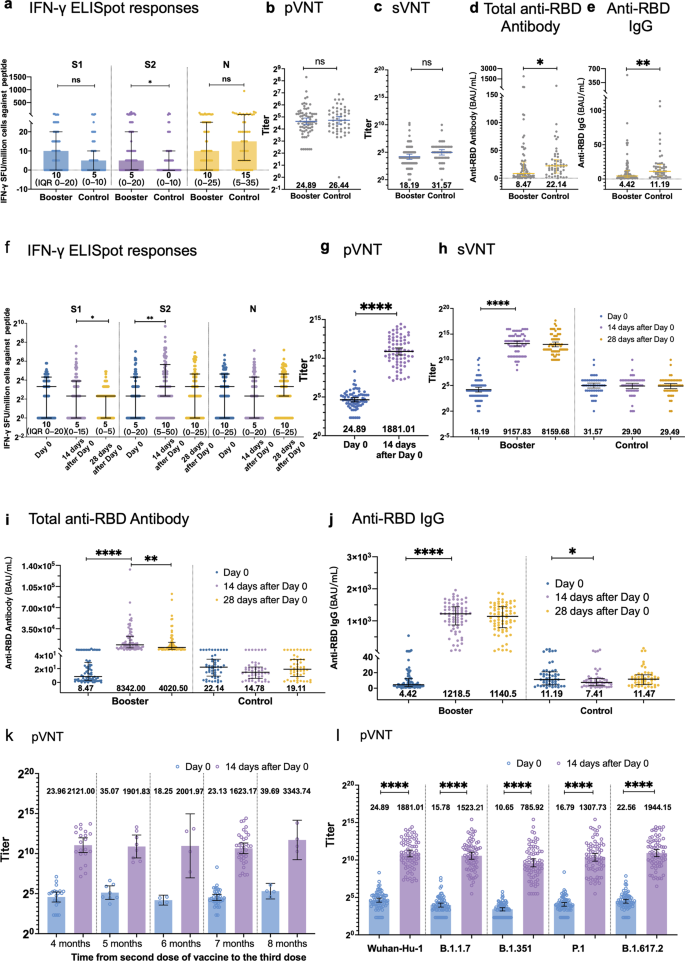
Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and Variants of Concern | Cell Research

COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study - The Lancet Infectious Diseases

Vaccines | Free Full-Text | The Antibody Response to the BNT162b2 mRNA COVID-19 Booster in Healthcare Workers: Association between the IgG Antibody Titers and Anthropometric and Body Composition Parameters

Crohn's & Colitis UK - We know there is some confusion between the third COVID-19 vaccine dose and the booster. This information helps to explain some of the differences. Vaccination is the

COVID-19 Vaccine Makers Plan for Annual Boosters, but It's Not Clear They'll Be Needed | Vaccination | JAMA | JAMA Network

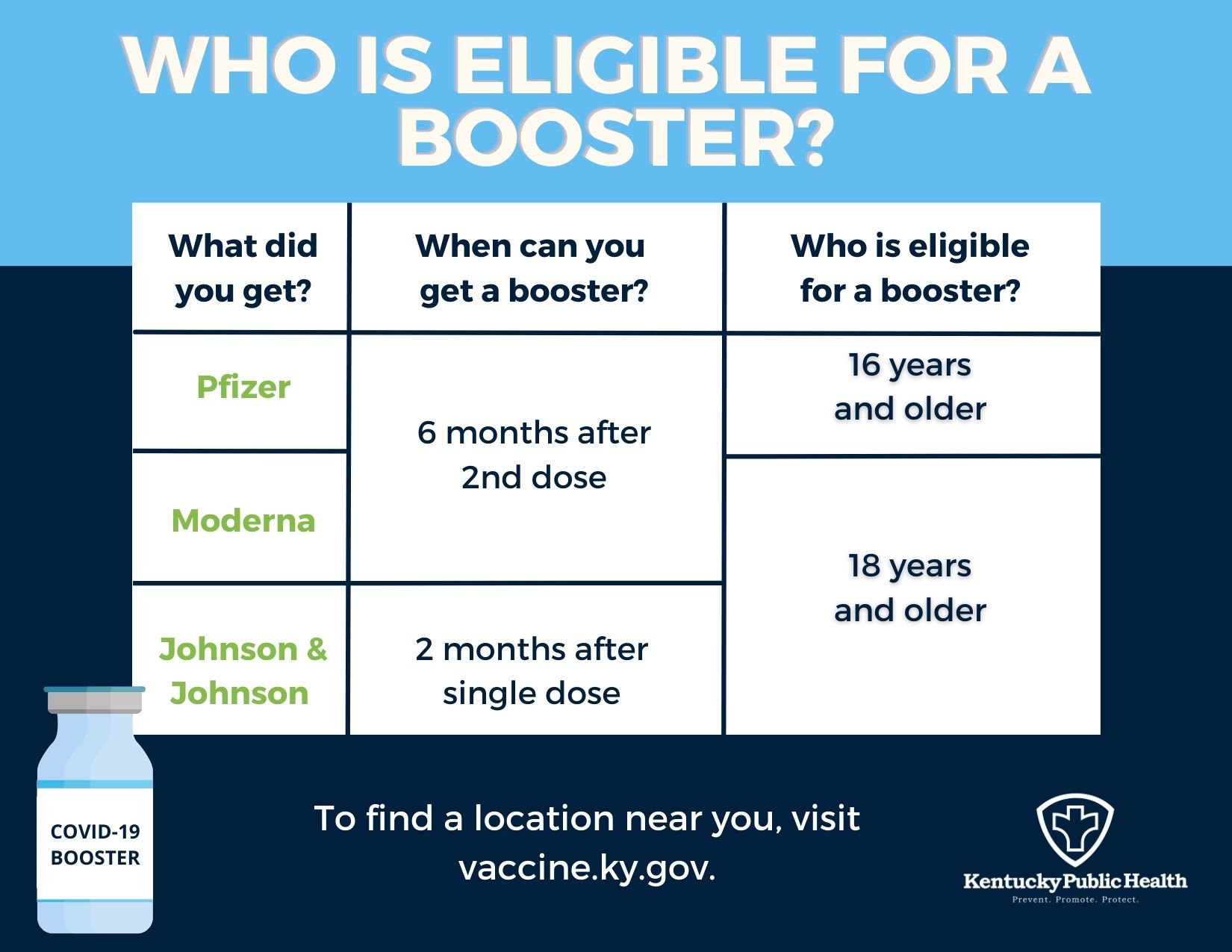
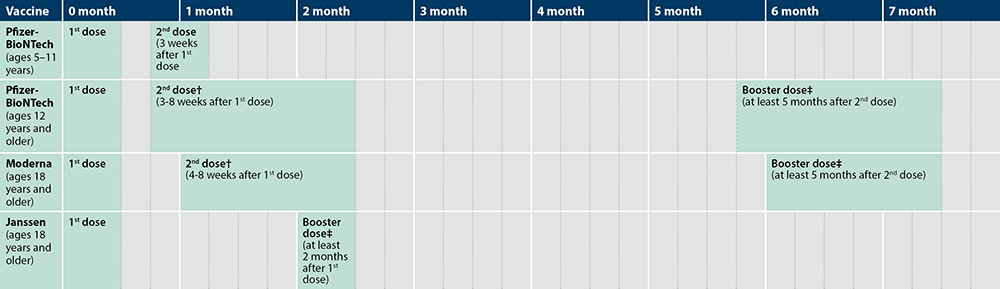
CDC on Twitter: "2) People who received a Pfizer-BioNTech #COVID19 vaccine for their primary series can now get an mRNA booster shot 5 months after they complete their series. 3) Everyone ages
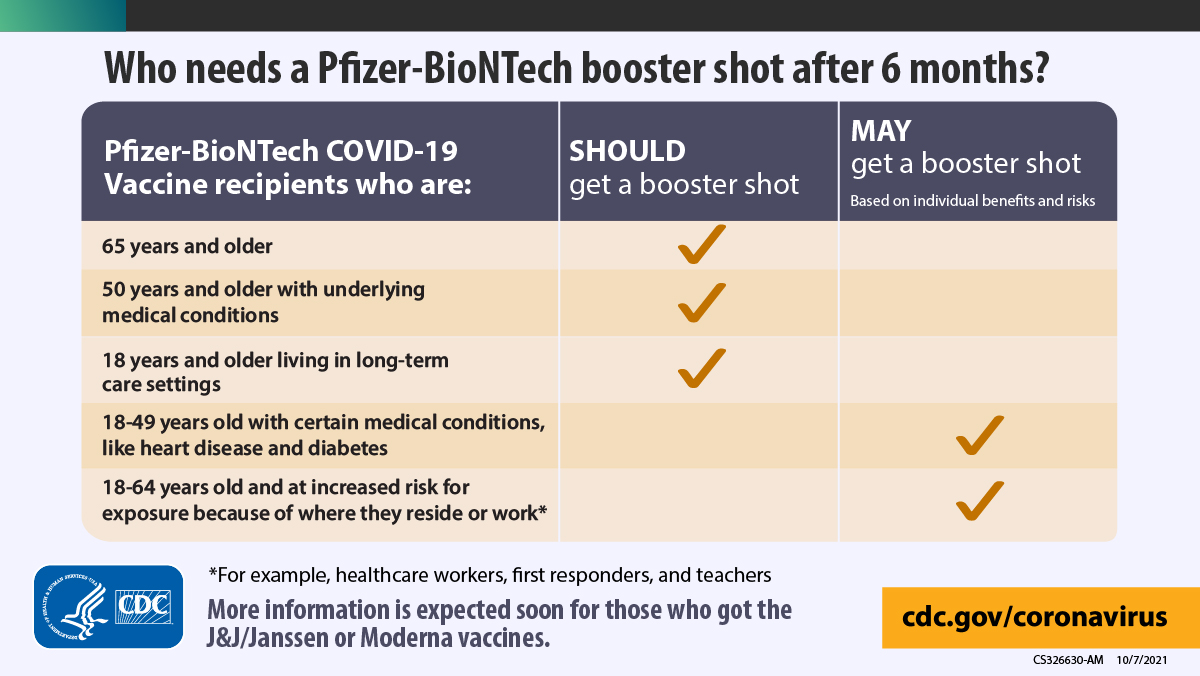
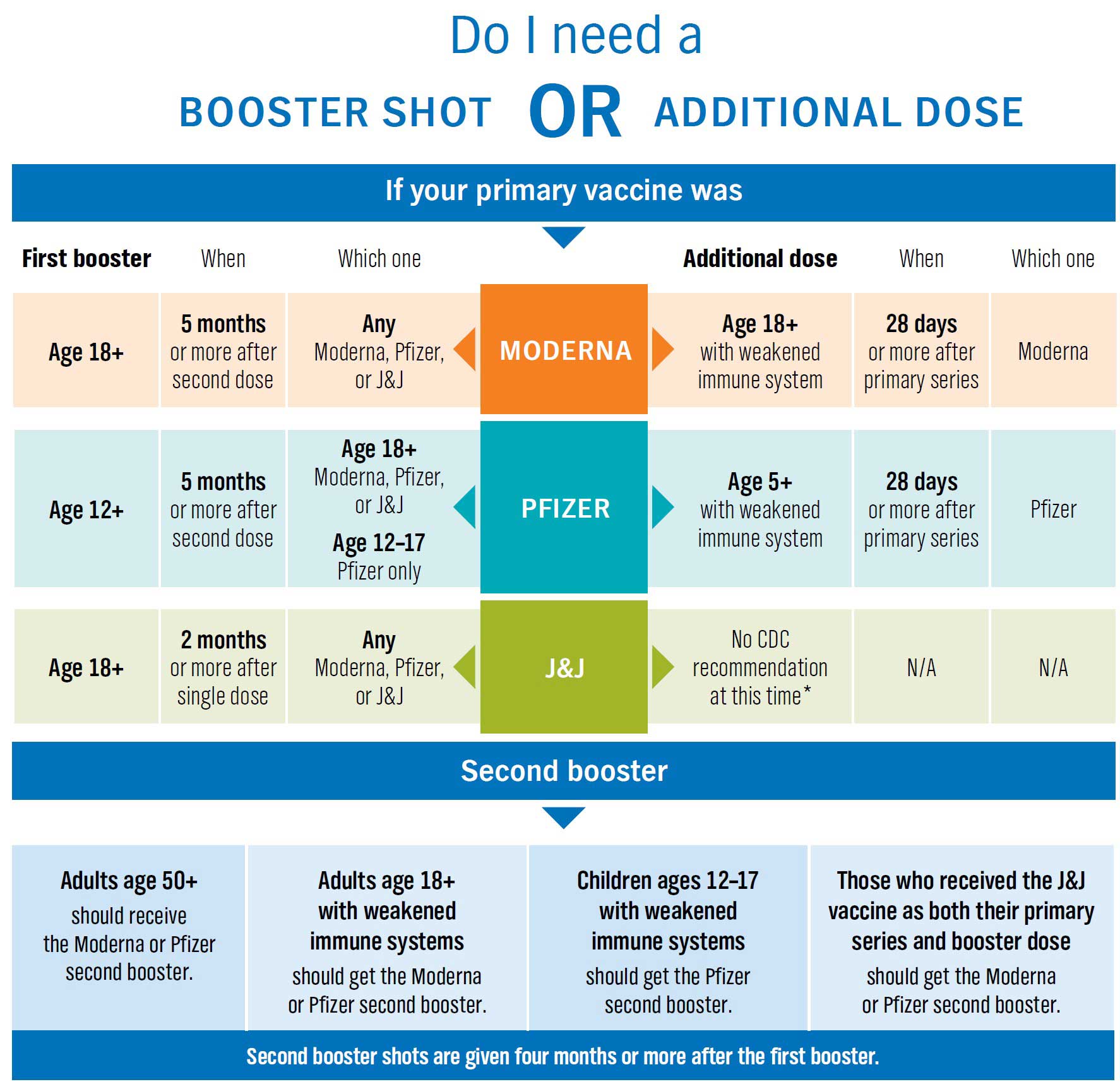
CDC on Twitter: "Wondering if you need a #COVID19 booster shot? If you received your second dose of the Pfizer-BioNTech COVID-19 Vaccine at least 6 months ago, use this chart to determine
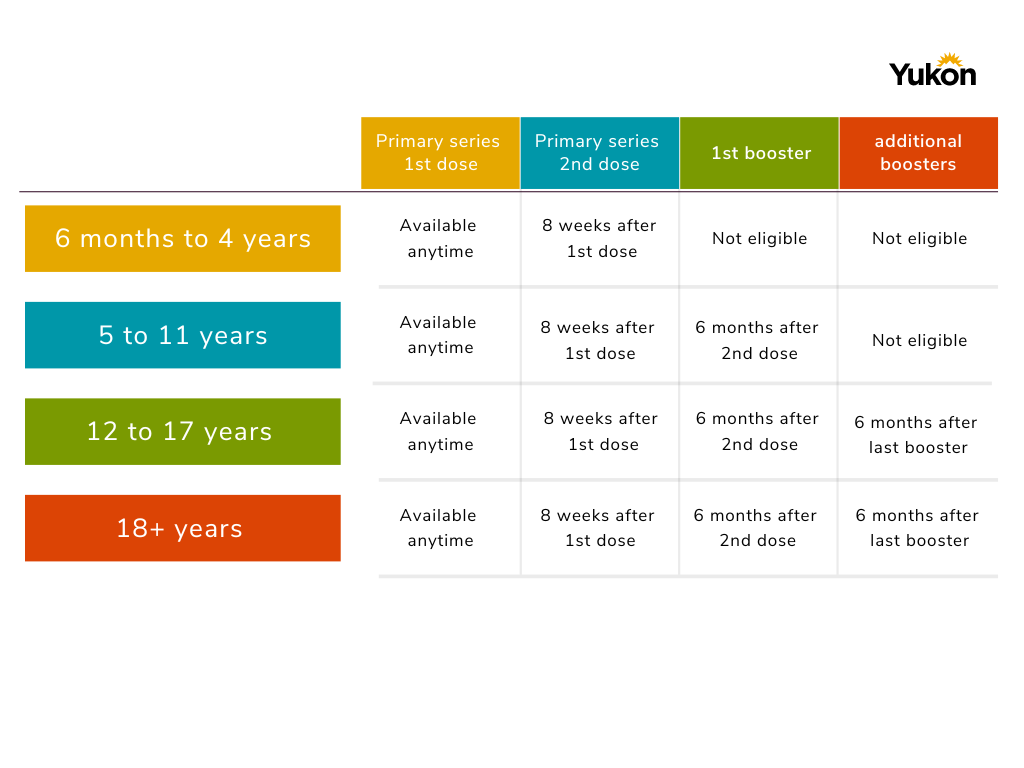
CDC updates COVID-19 vaccine recommended wait time to eight weeks for some groups | Children's Minnesota