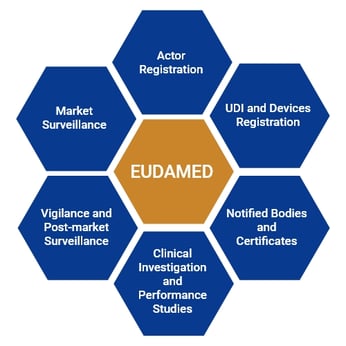
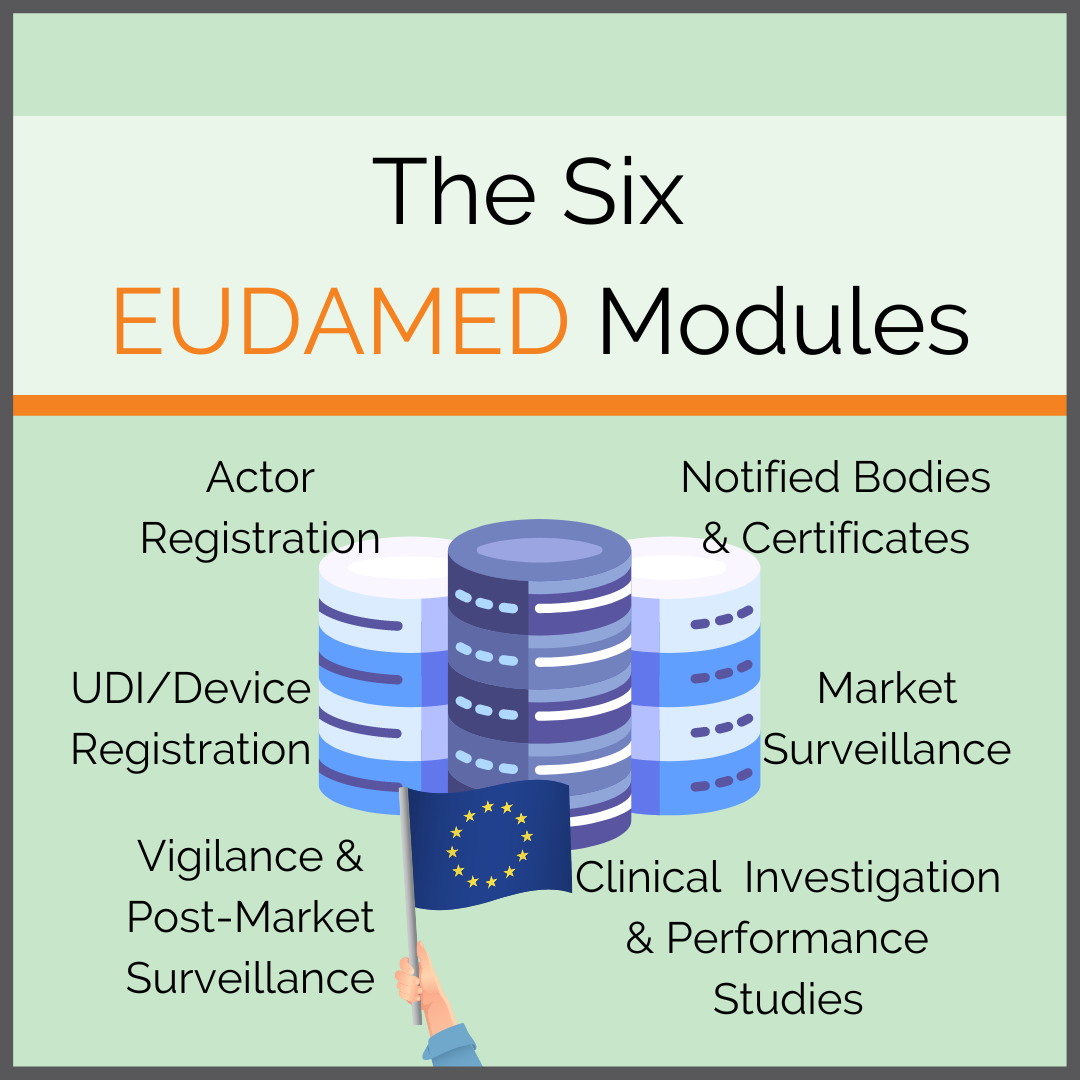
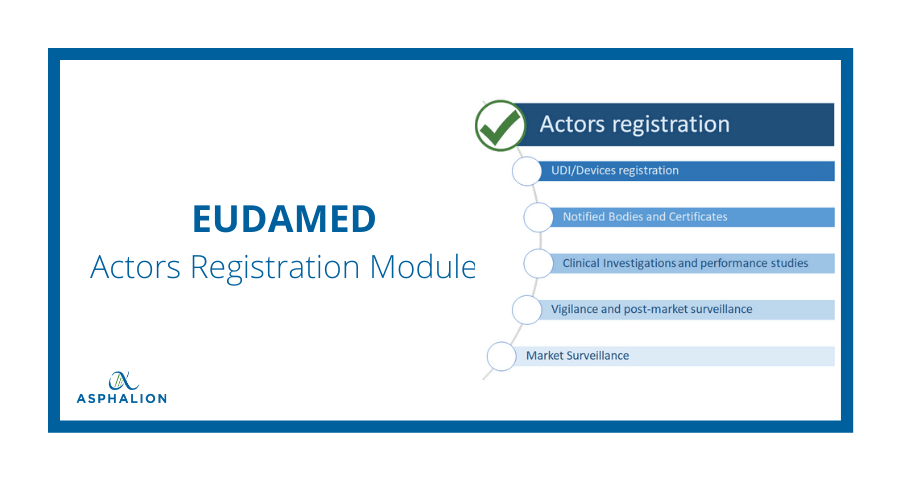
European Commission issues FAQ for medical device manufacturers on Eudamed Actor Module registration

Meditrial - Despite the decision of the #europeancommission to delay the activation of #EUDAMED till May 2022, #RAPS reports the Commission will begin making some #EUDAMED modules available next year, including the